Adjusting Some Properties of Poly(methacrylic acid) (Nano)Composite Hydrogels by Means of Silicon-Containing Inorganic Fillers
- PMID: 36142243
- PMCID: PMC9499409
- DOI: 10.3390/ijms231810320
Adjusting Some Properties of Poly(methacrylic acid) (Nano)Composite Hydrogels by Means of Silicon-Containing Inorganic Fillers
Abstract
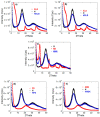
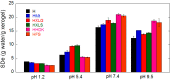
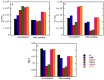
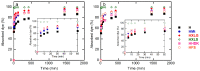
The present work aims to show how the main properties of poly(methacrylic acid) (PMAA) hydrogels can be engineered by means of several silicon-based fillers (Laponite XLS/XLG, montmorillonite (Mt), pyrogenic silica (PS)) employed at 10 wt% concentration based on MAA. Various techniques (FT-IR, XRD, TGA, SEM, TEM, DLS, rheological measurements, UV-VIS) were used to comparatively study the effect of these fillers, in correlation with their characteristics, upon the structure and swelling, viscoelastic, and water decontamination properties of (nano)composite hydrogels. The experiments demonstrated that the nanocomposite hydrogel morphology was dictated by the way the filler particles dispersed in water. The equilibrium swelling degree (SDe) depended on both the pH of the environment and the filler nature. At pH 1.2, a slight crosslinking effect of the fillers was evidenced, increasing in the order Mt < Laponite < PS. At pH > pKaMAA (pH 5.4; 7.4; 9.5), the Laponite/Mt-containing hydrogels displayed a higher SDe as compared to the neat one, while at pH 7.4/9.5 the PS-filled hydrogels surprisingly displayed the highest SDe. Rheological measurements on as-prepared hydrogels showed that the filler addition improved the mechanical properties. After equilibrium swelling at pH 5.4, G’ and G” depended on the filler, the Laponite-reinforced hydrogels proving to be the strongest. The (nano)composite hydrogels synthesized displayed filler-dependent absorption properties of two cationic dyes used as model water pollutants, Laponite XLS-reinforced hydrogel demonstrating both the highest absorption rate and absorption capacity. Besides wastewater purification, the (nano)composite hydrogels described here may also find applications in the pharmaceutical field as devices for the controlled release of drugs.
Keywords: Laponite; hydrogel; montmorillonite; nanocomposites; poly(methacrylic acid); pyrogenic silica; water decontamination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Egbo M.K. A Fundamental Review on Composite Materials and Some of Their Applications in Biomedical Engineering. J. King Saud Univ. Eng. Sci. 2020;33:557–568. doi: 10.1016/j.jksues.2020.07.007. - DOI
-
- Tishkevich D.I., Vorobjova A.I., Trukhanov A.V. Thermal Stability of Nano-Crystalline Nickel Electrodeposited into Porous Alumina. Solid State Phenom. 2020;299:281–286. doi: 10.4028/www.scientific.net/SSP.299.281. - DOI
-
- Tishkevich D.I., Grabchikov S.S., Grabchikova E.A., Vasin D.S., Yakushevich A.S., Vinnik D.A., Zubar T.I., Kalagin I.V., Yakimchuk D.V., Trukhanov A.V. Modeling of Paths and Energy Losses of High-Energy Ions in Single-Layered and Multilayered Materials. IOP Conf. Ser. Mater. Sci. Eng. 2020;848:012089. doi: 10.1088/1757-899X/848/1/012089. - DOI
-
- Tishkevich D.I., Vorobjova A.I., Vinnik D.A. Formation and Corrosion Behavior of Nickel/Alumina Nanocomposites. Solid State Phenom. 2020;299:100–106. doi: 10.4028/www.scientific.net/SSP.299.100. - DOI
-
- Tishkevich D.I., Zubar T.I., Zhaludkevich A.L., Razanau I.U., Vershinina T.N., Bondaruk A.A., Zheleznova E.K., Dong M., Hanfi M.Y., Sayyed M.I., et al. Isostatic Hot Pressed W–Cu Composites with Nanosized Grain Boundaries: Microstructure, Structure and Radiation Shielding Efficiency against Gamma Rays. Nanomaterials. 2022;12:1642. doi: 10.3390/nano12101642. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

