Hesperetin from Root Extract of Clerodendrum petasites S. Moore Inhibits SARS-CoV-2 Spike Protein S1 Subunit-Induced NLRP3 Inflammasome in A549 Lung Cells via Modulation of the Akt/MAPK/AP-1 Pathway
- PMID: 36142258
- PMCID: PMC9498987
- DOI: 10.3390/ijms231810346
Hesperetin from Root Extract of Clerodendrum petasites S. Moore Inhibits SARS-CoV-2 Spike Protein S1 Subunit-Induced NLRP3 Inflammasome in A549 Lung Cells via Modulation of the Akt/MAPK/AP-1 Pathway
Abstract
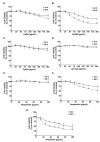
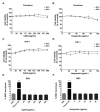
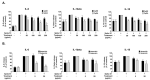
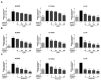
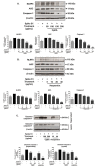
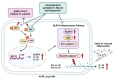
Inhibition of inflammatory responses from the spike glycoprotein of SARS-CoV-2 (Spike) by targeting NLRP3 inflammasome has recently been developed as an alternative form of supportive therapy besides the traditional anti-viral approaches. Clerodendrum petasites S. Moore (C. petasites) is a Thai traditional medicinal plant possessing antipyretic and anti-inflammatory activities. In this study, C. petasites ethanolic root extract (CpEE) underwent solvent-partitioned extraction to obtain the ethyl acetate fraction of C. petasites (CpEA). Subsequently, C. petasites extracts were determined for the flavonoid contents and anti-inflammatory properties against spike induction in the A549 lung cells. According to the HPLC results, CpEA significantly contained higher amounts of hesperidin and hesperetin flavonoids than CpEE (p < 0.05). A549 cells were then pre-treated with either C. petasites extracts or its active flavonoids and were primed with 100 ng/mL of spike S1 subunit (Spike S1) and determined for the anti-inflammatory properties. The results indicate that CpEA (compared with CpEE) and hesperetin (compared with hesperidin) exhibited greater anti-inflammatory properties upon Spike S1 induction through a significant reduction in IL-6, IL-1β, and IL-18 cytokine releases in A549 cells culture supernatant (p < 0.05). Additionally, CpEA and hesperetin significantly inhibited the Spike S1-induced inflammatory gene expressions (NLRP3, IL-1β, and IL-18, p < 0.05). Mechanistically, CpEA and hesperetin attenuated inflammasome machinery protein expressions (NLRP3, ASC, and Caspase-1), as well as inactivated the Akt/MAPK/AP-1 pathway. Overall, our findings could provide scientific-based evidence to support the use of C. petasites and hesperetin in the development of supportive therapies for the prevention of COVID-19-related chronic inflammation.
Keywords: COVID-19; Clerodendrum petasites; NLRP3 inflammasome; anti-inflammation; chronic inflammation; hesperetin; spike glycoprotein S1.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID.Front Med (Lausanne). 2023 Jan 9;9:1072056. doi: 10.3389/fmed.2022.1072056. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698809 Free PMC article.
-
Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway.Nutrients. 2022 Jun 30;14(13):2738. doi: 10.3390/nu14132738. Nutrients. 2022. PMID: 35807916 Free PMC article.
-
Anthocyanin-Rich Fraction from Kum Akha Black Rice Attenuates NLRP3 Inflammasome-Driven Lung Inflammation In Vitro and In Vivo.Nutrients. 2025 Mar 28;17(7):1186. doi: 10.3390/nu17071186. Nutrients. 2025. PMID: 40218944 Free PMC article.
-
Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation.Inflamm Res. 2022 Mar;71(3):293-307. doi: 10.1007/s00011-022-01540-y. Epub 2022 Feb 3. Inflamm Res. 2022. PMID: 35113170 Free PMC article. Review.
-
[Role of inflammasome NLRP3 in the pathophysiology of viral infections: A focus on SARS-CoV-2 infection].Med Sci (Paris). 2022 Jun-Jul;38(6-7):545-552. doi: 10.1051/medsci/2022080. Epub 2022 Jun 29. Med Sci (Paris). 2022. PMID: 35766852 Review. French.
Cited by
-
Protective Effects of Proanthocyanidin-Rich Fraction from Red Rice Germ and Bran on Lung Cell Inflammation via Inhibition of NF-κB/NLRP3 Inflammasome Pathway.Nutrients. 2023 Aug 30;15(17):3793. doi: 10.3390/nu15173793. Nutrients. 2023. PMID: 37686825 Free PMC article.
-
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein S1 Induces Methylglyoxal-Derived Hydroimidazolone/Receptor for Advanced Glycation End Products (MG-H1/RAGE) Activation to Promote Inflammation in Human Bronchial BEAS-2B Cells.Int J Mol Sci. 2023 Oct 3;24(19):14868. doi: 10.3390/ijms241914868. Int J Mol Sci. 2023. PMID: 37834316 Free PMC article.
-
Targeting MAPK Signaling: Loureirins A and B from Dracaena Loureiri Inhibit Epithelial-Mesenchymal Transition and Invasion in Non-Small Cell Lung Cancer Cell Lines.Life (Basel). 2025 Mar 3;15(3):396. doi: 10.3390/life15030396. Life (Basel). 2025. PMID: 40141741 Free PMC article.
-
Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID.Front Med (Lausanne). 2023 Jan 9;9:1072056. doi: 10.3389/fmed.2022.1072056. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698809 Free PMC article.
-
Suppression of inflammation-induced lung cancer cells proliferation and metastasis by exiguaflavanone A and exiguaflavanone B from Sophora exigua root extract through NLRP3 inflammasome pathway inhibition.Front Pharmacol. 2023 Nov 10;14:1243727. doi: 10.3389/fphar.2023.1243727. eCollection 2023. Front Pharmacol. 2023. PMID: 38026959 Free PMC article.
References
-
- Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., Noel A., Gunning S., Hatrick J., Hamilton S., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. - DOI - PMC - PubMed
-
- Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2020;218:1–11. doi: 10.1084/jem.20201707. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

