Does Pyroptosis Play a Role in Inflammasome-Related Disorders?
- PMID: 36142364
- PMCID: PMC9499396
- DOI: 10.3390/ijms231810453
Does Pyroptosis Play a Role in Inflammasome-Related Disorders?
Abstract
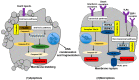
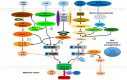
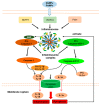
Inflammasomes are multiprotein complexes orchestrating intracellular recognition of endogenous and exogenous stimuli, cellular homeostasis, and cell death. Upon sensing of certain stimuli, inflammasomes typically activate inflammatory caspases that promote the production and release of the proinflammatory cytokines IL-1β, IL-1α, and IL-18 and induce a type of inflammatory cell death known as "pyroptosis". Pyroptosis is an important form of regulated cell death executed by gasdermin proteins, which is largely different from apoptosis and necrosis. Recently, several signaling pathways driving pyroptotic cell death, including canonical and noncanonical inflammasome activation, as well as caspase-3-dependent pathways, have been reported. While much evidence exists that pyroptosis is involved in the development of several inflammatory diseases, its contribution to inflammasome-related disorders (IRDs) has not been fully clarified. This article reviews molecular mechanisms leading to pyroptosis, and attempts to provide evidence for its possible role in inflammasome-related disorders, including NLR pyrin domain containing 3 (NLRP3) inflammasome disease, NLR containing a caspase recruitment domain 4 (NLRC4) inflammasome disease, and pyrin inflammasome disease. Although the specific mechanism needs further investigations, these studies have uncovered the role of pyroptosis in inflammasome-related disorders and may open new avenues for future therapeutic interventions.
Keywords: RCD; apoptosis; inflammasome disease; inflammasomes; necroptosis; pyroptosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases.J Cell Physiol. 2020 Apr;235(4):3207-3221. doi: 10.1002/jcp.29268. Epub 2019 Oct 17. J Cell Physiol. 2020. PMID: 31621910 Review.
-
NLRP3 inflammasome activation and cell death.Cell Mol Immunol. 2021 Sep;18(9):2114-2127. doi: 10.1038/s41423-021-00740-6. Epub 2021 Jul 28. Cell Mol Immunol. 2021. PMID: 34321623 Free PMC article. Review.
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
The intersection of cell death and inflammasome activation.Cell Mol Life Sci. 2016 Jun;73(11-12):2349-67. doi: 10.1007/s00018-016-2205-2. Epub 2016 Apr 11. Cell Mol Life Sci. 2016. PMID: 27066895 Free PMC article. Review.
Cited by
-
MNDA, a PYHIN factor involved in transcriptional regulation and apoptosis control in leukocytes.Front Immunol. 2024 Apr 12;15:1395035. doi: 10.3389/fimmu.2024.1395035. eCollection 2024. Front Immunol. 2024. PMID: 38680493 Free PMC article. Review.
-
A morphology and secretome map of pyroptosis.Mol Biol Cell. 2025 Jun 1;36(6):ar63. doi: 10.1091/mbc.E25-03-0119. Epub 2025 Apr 9. Mol Biol Cell. 2025. PMID: 40202832
-
Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells.Nutrients. 2024 Sep 1;16(17):2929. doi: 10.3390/nu16172929. Nutrients. 2024. PMID: 39275245 Free PMC article.
-
NLRX1 knockdown attenuates pro-apoptotic signaling and cell death in pulmonary hyperoxic acute injury.Sci Rep. 2023 Mar 1;13(1):3441. doi: 10.1038/s41598-023-28206-x. Sci Rep. 2023. PMID: 36859435 Free PMC article.
-
Chemical modulation of gasdermin D activity: Therapeutic implications and consequences.Semin Immunol. 2023 Nov;70:101845. doi: 10.1016/j.smim.2023.101845. Epub 2023 Oct 8. Semin Immunol. 2023. PMID: 37806032 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

