Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites
- PMID: 36142380
- PMCID: PMC9499569
- DOI: 10.3390/ijms231810454
Effects of Sterilization and Hydrolytic Degradation on the Structure, Morphology and Compressive Strength of Polylactide-Hydroxyapatite Composites
Abstract
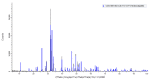
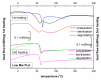
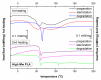
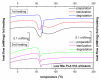
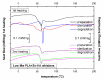
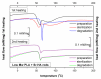
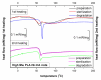
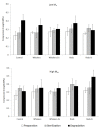
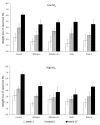
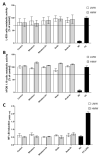
Composites based on polylactide (PLA) and hydroxyapatite (HA) were prepared using a thermally induced phase separation method. In the experimental design, the PLA with low weight-average molar mass (Mw) and high Mw were tested with the inclusion of HA synthesized as whiskers or hexagonal rods. In addition, the structure of HA whiskers was doped with Zn, whereas hexagonal rods were mixed with Sr salt. The composites were sterilized and then incubated in phosphate-buffered saline for 12 weeks at 37 °C, followed by characterization of pore size distribution, molecular properties, density and mechanical strength. Results showed a substantial reduction of PLA Mw for both polymers due to the preparation of composites, their sterilization and incubation. The distribution of pore size effectively increased after the degradation process, whereas the sterilization, furthermore, had an impact on pore size distribution depending on HA added. The inclusion of HA reduced to some extent the degradation of PLA quantitatively in the weight loss in vitro compared to the control without HA. All produced materials showed no cytotoxicity when validated against L929 mouse skin fibroblasts and hFOB 1.19 human osteoblasts. The lack of cytotoxicity was accompanied by the immunocompatibility with human monocytic cells that were able to detect pyrogenic contaminants.
Keywords: PLA; biocomposites; hydrolytic degradation; hydroxyapatite; sterilization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Preparation and mechanical properties of carbon fiber reinforced hydroxyapatite/polylactide biocomposites.J Mater Sci Mater Med. 2009 Nov;20(11):2259-65. doi: 10.1007/s10856-009-3785-2. Epub 2009 Jun 2. J Mater Sci Mater Med. 2009. PMID: 19488680
-
Improved mechanical properties of hydroxyapatite whisker-reinforced poly(L-lactic acid) scaffold by surface modification of hydroxyapatite.Mater Sci Eng C Mater Biol Appl. 2014 Feb 1;35:190-4. doi: 10.1016/j.msec.2013.11.008. Epub 2013 Nov 14. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24411368
-
Cell responses and hemocompatibility of g-HA/PLA composites.Sci China Life Sci. 2011 Apr;54(4):366-71. doi: 10.1007/s11427-011-4155-0. Epub 2011 Mar 17. Sci China Life Sci. 2011. PMID: 21416229
-
Impact of Zn-Modified Hydroxyapatite Whiskers on Physicochemical and Biological Properties of Poly(ε-Caprolactone) Composites Intended for Implantable Medical Devices.J Biomed Mater Res B Appl Biomater. 2025 May;113(5):e35586. doi: 10.1002/jbm.b.35586. J Biomed Mater Res B Appl Biomater. 2025. PMID: 40271807
-
Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109960. doi: 10.1016/j.msec.2019.109960. Epub 2019 Jul 6. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31500051
Cited by
-
Dual Modification of Porous Ca-P/PLA Composites with APTES and Alendronate Improves Their Mechanical Strength and Cytobiocompatibility towards Human Osteoblasts.Int J Mol Sci. 2022 Nov 18;23(22):14315. doi: 10.3390/ijms232214315. Int J Mol Sci. 2022. PMID: 36430791 Free PMC article.
References
-
- Van Houdt C.I.A., Gabbai-Armelin P.R., Lopez-Perez P.M., Ulrich D.J.O., Jansen J.A., Renno A.C.M., van den Beucken J.J.J.P. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018;8:15398. doi: 10.1038/s41598-018-33692-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

