Potent Chlorambucil-Platinum(IV) Prodrugs
- PMID: 36142383
- PMCID: PMC9499463
- DOI: 10.3390/ijms231810471
Potent Chlorambucil-Platinum(IV) Prodrugs
Abstract
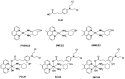
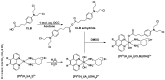
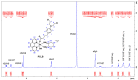
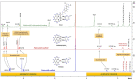
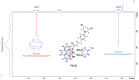
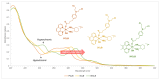
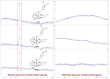
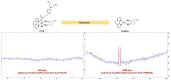
The DNA-alkylating derivative chlorambucil was coordinated in the axial position to atypical cytotoxic, heterocyclic, and non-DNA coordinating platinum(IV) complexes of type, [PtIV(HL)(AL)(OH)2](NO3)2 (where HL is 1,10-phenanthroline, 5-methyl-1,10-phenanthroline or 5,6-dimethyl-1,10-phenanthroline, AL is 1S,2S-diaminocyclohexane). The resultant platinum(IV)-chlorambucil prodrugs, PCLB, 5CLB, and 56CLB, were characterized using high-performance liquid chromatography, nuclear magnetic resonance, ultraviolet-visible, circular dichroism spectroscopy, and electrospray ionization mass spectrometry. The prodrugs displayed remarkable antitumor potential across multiple human cancer cell lines compared to chlorambucil, cisplatin, oxaliplatin, and carboplatin, as well as their platinum(II) precursors, PHENSS, 5MESS, and 56MESS. Notably, 56CLB was exceptionally potent in HT29 colon, Du145 prostate, MCF10A breast, MIA pancreas, H460 lung, A2780, and ADDP ovarian cell lines, with GI50 values ranging between 2.7 and 21 nM. Moreover, significant production of reactive oxygen species was detected in HT29 cells after treatment with PCLB, 5CLB, and 56CLB up to 72 h compared to chlorambucil and the platinum(II) and (IV) precursors.
Keywords: 56MESS; 5MESS; DNA; PHENSS; ROS; chlorambucil; cytotoxicity; platinum(II); platinum(IV).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Paprocka R., Wiese-Szadkowska M., Janciauskiene S., Kosmalski T., Kulik M., Helmin-Basa A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022;452:214307. doi: 10.1016/j.ccr.2021.214307. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

