Role of Circular RNAs in Pulmonary Fibrosis
- PMID: 36142402
- PMCID: PMC9504269
- DOI: 10.3390/ijms231810493
Role of Circular RNAs in Pulmonary Fibrosis
Abstract
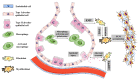
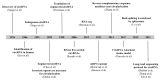
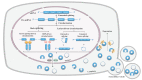
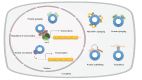
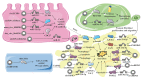
Pulmonary fibrosis is a chronic progressive form of interstitial lung disease, characterized by the histopathological pattern of usual interstitial pneumonia. Apart from aberrant alterations of protein-coding genes, dysregulation of non-coding RNAs, including microRNAs, long non-coding RNAs, and circular RNAs (circRNAs), is crucial to the initiation and progression of pulmonary fibrosis. CircRNAs are single-stranded RNAs that form covalently closed loops without 5' caps and 3' tails. Different from canonical splicing of mRNA, they are produced from the back-splicing of precursor mRNAs and have unique biological functions, as well as potential biomedical implications. They function as important gene regulators through multiple actions, including sponging microRNAs and proteins, regulating transcription, and splicing, as well as protein-coding and translation in a cap-independent manner. This review comprehensively summarizes the alteration and functional role of circRNAs in pulmonary fibrosis, with a focus on the involvement of the circRNA in the context of cell-specific pathophysiology. In addition, we discuss the diagnostic and therapeutic potential of targeting circRNA and their regulatory pathway mediators, which may facilitate the translation of recent advances from bench to bedside in the future.
Keywords: circRNA; pulmonary fibrosis; silicosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulatory circular RNAs in viral diseases: applications in diagnosis and therapy.RNA Biol. 2023 Jan;20(1):847-858. doi: 10.1080/15476286.2023.2272118. Epub 2023 Oct 26. RNA Biol. 2023. PMID: 37882652 Free PMC article. Review.
-
Potential regulatory role of circular RNA in idiopathic pulmonary fibrosis.Int J Mol Med. 2018 Dec;42(6):3256-3268. doi: 10.3892/ijmm.2018.3892. Epub 2018 Sep 24. Int J Mol Med. 2018. PMID: 30272257 Free PMC article.
-
The expanding regulatory mechanisms and cellular functions of circular RNAs.Nat Rev Mol Cell Biol. 2020 Aug;21(8):475-490. doi: 10.1038/s41580-020-0243-y. Epub 2020 May 4. Nat Rev Mol Cell Biol. 2020. PMID: 32366901 Review.
-
Circular RNAs with protein-coding ability in oncogenesis.Biochim Biophys Acta Rev Cancer. 2023 Jul;1878(4):188909. doi: 10.1016/j.bbcan.2023.188909. Epub 2023 May 10. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37172651 Review.
-
Molecular mechanisms of circular RNA translation.Exp Mol Med. 2024 Jun;56(6):1272-1280. doi: 10.1038/s12276-024-01220-3. Epub 2024 Jun 14. Exp Mol Med. 2024. PMID: 38871818 Free PMC article. Review.
Cited by
-
Deciphering the multifaceted role of circular RNA in aging: from molecular mechanisms to therapeutic potentials.Noncoding RNA Res. 2025 May 30;14:129-144. doi: 10.1016/j.ncrna.2025.05.015. eCollection 2025 Oct. Noncoding RNA Res. 2025. PMID: 40585063 Free PMC article. Review.
-
Emerging roles of noncoding RNAs in idiopathic pulmonary fibrosis.Cell Death Discov. 2024 Oct 21;10(1):443. doi: 10.1038/s41420-024-02170-5. Cell Death Discov. 2024. PMID: 39433746 Free PMC article. Review.
-
Senescent AECⅡ and the implication for idiopathic pulmonary fibrosis treatment.Front Pharmacol. 2022 Nov 15;13:1059434. doi: 10.3389/fphar.2022.1059434. eCollection 2022. Front Pharmacol. 2022. PMID: 36457712 Free PMC article. Review.
-
Exploring the Interplay between Cellular Senescence, Immunity, and Fibrosing Interstitial Lung Diseases: Challenges and Opportunities.Int J Mol Sci. 2024 Jul 10;25(14):7554. doi: 10.3390/ijms25147554. Int J Mol Sci. 2024. PMID: 39062798 Free PMC article. Review.
-
Diagnostic value of miR-34a in Mycoplasma pneumoniae pneumonia in children and its correlation with rehabilitation effect.J Cardiothorac Surg. 2024 Sep 2;19(1):507. doi: 10.1186/s13019-024-02992-5. J Cardiothorac Surg. 2024. PMID: 39223566 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

