MicroRNAs, Stem Cells in Bipolar Disorder, and Lithium Therapeutic Approach
- PMID: 36142403
- PMCID: PMC9502703
- DOI: 10.3390/ijms231810489
MicroRNAs, Stem Cells in Bipolar Disorder, and Lithium Therapeutic Approach
Abstract
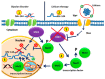
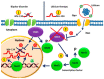
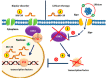
Bipolar disorder (BD) is a severe, chronic, and disabling neuropsychiatric disorder characterized by recurrent mood disturbances (mania/hypomania and depression, with or without mixed features) and a constellation of cognitive, psychomotor, autonomic, and endocrine abnormalities. The etiology of BD is multifactorial, including both biological and epigenetic factors. Recently, microRNAs (miRNAs), a class of epigenetic regulators of gene expression playing a central role in brain development and plasticity, have been related to several neuropsychiatric disorders, including BD. Moreover, an alteration in the number/distribution and differentiation potential of neural stem cells has also been described, significantly affecting brain homeostasis and neuroplasticity. This review aimed to evaluate the most reliable scientific evidence on miRNAs as biomarkers for the diagnosis of BD and assess their implications in response to mood stabilizers, such as lithium. Neural stem cell distribution, regulation, and dysfunction in the etiology of BD are also dissected.
Keywords: bipolar disorder (BD); lithium; microRNA; molecular mechanisms; neural stem cells; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Merikangas K.R., Jin R., He J.-P., Kessler R.C., Lee S., Sampson N.A., Viana M.C., Andrade L.H., Hu C., Karam E.G., et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. - DOI - PMC - PubMed
-
- Raychaudhuri S., Plenge R.M., Rossin E.J., Ng A.C.Y., Purcell S.M., Sklar P., Scolnick E.M., Xavier R.J., Altshuler D., Daly M.J., et al. Identifying Relationships among Genomic Disease Regions: Predicting Genes at Pathogenic SNP Associations and Rare Deletions. PLoS Genet. 2009;5:e1000534. doi: 10.1371/journal.pgen.1000534. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

