Combined PARP and Dual Topoisomerase Inhibition Potentiates Genome Instability and Cell Death in Ovarian Cancer
- PMID: 36142413
- PMCID: PMC9505822
- DOI: 10.3390/ijms231810503
Combined PARP and Dual Topoisomerase Inhibition Potentiates Genome Instability and Cell Death in Ovarian Cancer
Abstract
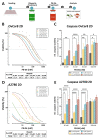
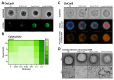
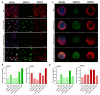
Although ovarian cancer is a rare disease, it constitutes the fifth leading cause of cancer death among women. It is of major importance to develop new therapeutic strategies to improve survival. Combining P8-D6, a novel dual topoisomerase inhibitor with exceptional anti-tumoral properties in ovarian cancer and compounds in preclinical research, and olaparib, a PARP inhibitor targeting DNA damage repair, is a promising approach. P8-D6 induces DNA damage that can be repaired by base excision repair or homologous recombination in which PARP plays a major role. This study analyzed benefits of combining P8-D6 and olaparib treatment in 2D and 3D cultures with ovarian cancer cells. Measurement of viability, cytotoxicity and caspase activity were used to assess therapy efficacy and to calculate the combination index (CI). Further DNA damage was quantified using the biomarkers RAD51 and γH2A.X. The combinational treatment led to an increased caspase activity and reduced viability. CI values partially show synergisms in combinations at 100 nM and 500 nM P8-D6. More DNA damage accumulated, and spheroids lost their membrane integrity due to the combinational treatment. While maintaining the same therapy efficacy as single-drug therapy, doses of P8-D6 and olaparib can be reduced in combinational treatments. Synergisms can be seen in some tested combinations. In summary, the combination therapy indicates benefits and acts synergistic at 100 nM and 500 nM P8-D6.
Keywords: PARP inhibitor; dual topoisomerase inhibitor; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Entinostat, a selective HDAC1/2 inhibitor, potentiates the effects of olaparib in homologous recombination proficient ovarian cancer.Gynecol Oncol. 2021 Jul;162(1):163-172. doi: 10.1016/j.ygyno.2021.04.015. Epub 2021 Apr 16. Gynecol Oncol. 2021. PMID: 33867143 Free PMC article.
-
The Poly (ADP-ribose) polymerase inhibitor olaparib and pan-ErbB inhibitor neratinib are highly synergistic in HER2 overexpressing epithelial ovarian carcinoma in vitro and in vivo.Gynecol Oncol. 2023 Mar;170:172-178. doi: 10.1016/j.ygyno.2023.01.015. Epub 2023 Jan 25. Gynecol Oncol. 2023. PMID: 36706643 Free PMC article.
-
The BET inhibitor INCB054329 reduces homologous recombination efficiency and augments PARP inhibitor activity in ovarian cancer.Gynecol Oncol. 2018 Jun;149(3):575-584. doi: 10.1016/j.ygyno.2018.03.049. Epub 2018 Mar 20. Gynecol Oncol. 2018. PMID: 29567272 Free PMC article.
-
Clinical Application of Poly(ADP-Ribose) Polymerase Inhibitors in High-Grade Serous Ovarian Cancer.Oncologist. 2016 May;21(5):586-93. doi: 10.1634/theoncologist.2015-0438. Epub 2016 Mar 28. Oncologist. 2016. PMID: 27022037 Free PMC article. Review.
-
Using PARP Inhibitors in the Treatment of Patients With Ovarian Cancer.Curr Treat Options Oncol. 2018 Nov 15;19(12):1. doi: 10.1007/s11864-018-0572-7. Curr Treat Options Oncol. 2018. PMID: 30535808 Free PMC article. Review.
Cited by
-
The Implication of Topoisomerase II Inhibitors in Synthetic Lethality for Cancer Therapy.Pharmaceuticals (Basel). 2023 Jan 9;16(1):94. doi: 10.3390/ph16010094. Pharmaceuticals (Basel). 2023. PMID: 36678591 Free PMC article. Review.
-
Diagnostic and therapeutic advances for HER2-expressing or amplified gynecologic cancers.Gynecol Oncol. 2025 Apr;195:152-164. doi: 10.1016/j.ygyno.2025.03.011. Epub 2025 Mar 20. Gynecol Oncol. 2025. PMID: 40117942 Free PMC article. Review.
-
Transcriptomic Analysis of CRISPR/Cas9-Mediated PARP1-Knockout Cells under the Influence of Topotecan and TDP1 Inhibitor.Int J Mol Sci. 2023 Mar 7;24(6):5148. doi: 10.3390/ijms24065148. Int J Mol Sci. 2023. PMID: 36982223 Free PMC article.
-
Dual Topoisomerase Inhibitor Is Highly Potent and Improves Antitumor Response to Radiotherapy in Cervical Carcinoma.Int J Mol Sci. 2025 Mar 21;26(7):2829. doi: 10.3390/ijms26072829. Int J Mol Sci. 2025. PMID: 40243435 Free PMC article.
-
In Vivo and In Vitro Pharmacokinetic Studies of a Dual Topoisomerase I/II Inhibitor.ACS Pharmacol Transl Sci. 2025 Mar 12;8(4):1050-1071. doi: 10.1021/acsptsci.4c00596. eCollection 2025 Apr 11. ACS Pharmacol Transl Sci. 2025. PMID: 40242581
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

