Interaction between Alzheimer's Disease and Cerebral Small Vessel Disease: A Review Focused on Neuroimaging Markers
- PMID: 36142419
- PMCID: PMC9499680
- DOI: 10.3390/ijms231810490
Interaction between Alzheimer's Disease and Cerebral Small Vessel Disease: A Review Focused on Neuroimaging Markers
Abstract
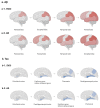
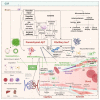
Alzheimer's disease (AD) is characterized by the presence of β-amyloid (Aβ) and tau, and subcortical vascular cognitive impairment (SVCI) is characterized by cerebral small vessel disease (CSVD). They are the most common causes of cognitive impairment in the elderly population. Concurrent CSVD burden is more commonly observed in AD-type dementia than in other neurodegenerative diseases. Recent developments in Aβ and tau positron emission tomography (PET) have enabled the investigation of the relationship between AD biomarkers and CSVD in vivo. In this review, we focus on the interaction between AD and CSVD markers and the clinical effects of these two markers based on molecular imaging studies. First, we cover the frequency of AD imaging markers, including Aβ and tau, in patients with SVCI. Second, we discuss the relationship between AD and CSVD markers and the potential distinct pathobiology of AD markers in SVCI compared to AD-type dementia. Next, we discuss the clinical effects of AD and CSVD markers in SVCI, and hemorrhagic markers in cerebral amyloid angiopathy. Finally, this review provides both the current challenges and future perspectives for SVCI.
Keywords: Alzheimer’s disease; cerebral small vessel disease; interaction; positron emission tomography; subcortical vascular cognitive impairment; tau; ß-Amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wenk G.L. Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry. 2003;64((Suppl. 9)):7–10. - PubMed
-
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
-
- Jack C.R., Jr., Wiste H.J., Therneau T.M., Weigand S.D., Knopman D.S., Mielke M.M., Lowe V.J., Vemuri P., Machulda M.M., Schwarz C.G., et al. Associations of Amyloid, Tau, and Neurodegeneration Biomarker Profiles With Rates of Memory Decline Among Individuals Without Dementia. Jama. 2019;321:2316–2325. doi: 10.1001/jama.2019.7437. - DOI - PMC - PubMed

