The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures
- PMID: 36142445
- PMCID: PMC9504018
- DOI: 10.3390/ijms231810533
The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures
Abstract
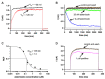
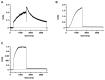
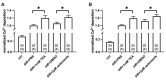
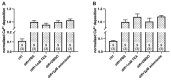
The hEag1 (Kv10.1) K+ channel is normally found in the brain, but it is ectopically expressed in tumor cells, including osteosarcoma. Based on the pivotal role of ion channels in osteogenesis, we tested whether pharmacological modulation of hEag1 may affect osteogenic differentiation of osteosarcoma cell lines. Using molecular biology (RT-PCR), electrophysiology (patch-clamp) and pharmacology (astemizole sensitivity, IC50 = 0.135 μM) we demonstrated that SaOS-2 osteosarcoma cells also express hEag1 channels. SaOS-2 cells also express to KCa1.1 K+ channels as shown by mRNA expression and paxilline sensitivity of the current. The inhibition of hEag1 (2 μM astemizole) or KCa1.1 (1 mM TEA) alone did not induce Ca2+ deposition in SaOS-2 cultures, however, these inhibitors, at identical concentrations, increased Ca2+ deposition evoked by the classical or pathological (inorganic phosphate, Pi) induction pathway without causing cytotoxicity, as reported by three completer assays (LDH release, MTT assay and SRB protein assay). We observed a similar effect of astemizole on Ca2+ deposition in MG-63 osteosarcoma cultures as well. We propose that the increase in the osteogenic stimuli-induced mineral matrix formation of osteosarcoma cell lines by inhibiting hEag1 may be a useful tool to drive terminal differentiation of osteosarcoma.
Keywords: Ca2+ deposition; Kv10.1; SaOS-2 osteosarcoma cells; hEag1 potassium channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
BKCa and hEag1 channels regulate cell proliferation and differentiation in human bone marrow-derived mesenchymal stem cells.J Cell Physiol. 2014 Feb;229(2):202-12. doi: 10.1002/jcp.24435. J Cell Physiol. 2014. PMID: 23881642
-
Mechanism of block of hEag1 K+ channels by imipramine and astemizole.J Gen Physiol. 2004 Oct;124(4):301-17. doi: 10.1085/jgp.200409041. Epub 2004 Sep 13. J Gen Physiol. 2004. PMID: 15365094 Free PMC article.
-
Overexpression of potassium channel ether à go-go in human osteosarcoma.Neoplasma. 2012;59(2):207-15. doi: 10.4149/neo_2012_027. Neoplasma. 2012. PMID: 22248279
-
Regulation of IGF-1-dependent cyclin D1 and E expression by hEag1 channels in MCF-7 cells: the critical role of hEag1 channels in G1 phase progression.Biochim Biophys Acta. 2011 May;1813(5):723-30. doi: 10.1016/j.bbamcr.2011.01.025. Epub 2011 Feb 16. Biochim Biophys Acta. 2011. PMID: 21315112
-
Astemizole: an old anti-histamine as a new promising anti-cancer drug.Anticancer Agents Med Chem. 2011 Mar;11(3):307-14. doi: 10.2174/187152011795347513. Anticancer Agents Med Chem. 2011. PMID: 21443504 Review.
Cited by
-
Unlocking the potential of stimuli-responsive biomaterials for bone regeneration.Front Pharmacol. 2024 Jul 31;15:1437457. doi: 10.3389/fphar.2024.1437457. eCollection 2024. Front Pharmacol. 2024. PMID: 39144636 Free PMC article. Review.
References
-
- Comes N., Serrano-Albarras A., Capera J., Serrano-Novillo C., Condom E., Ramon Y.C.S., Ferreres J.C., Felipe A. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim. Biophys. Acta. 2015;1848:2477–2492. doi: 10.1016/j.bbamem.2014.12.008. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

