Assembly of the Multi-Subunit Cytochrome bc1 Complex in the Yeast Saccharomyces cerevisiae
- PMID: 36142449
- PMCID: PMC9502982
- DOI: 10.3390/ijms231810537
Assembly of the Multi-Subunit Cytochrome bc1 Complex in the Yeast Saccharomyces cerevisiae
Abstract
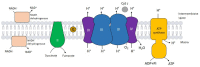
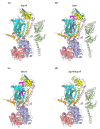
The cytochrome bc1 complex is an essential component of the mitochondrial respiratory chain of the yeast Saccharomyces cerevisiae. It is composed of ten protein subunits, three of them playing an important role in electron transfer and proton pumping across the inner mitochondrial membrane. Cytochrome b, the central component of this respiratory complex, is encoded by the mitochondrial genome, whereas all the other subunits are of nuclear origin. The assembly of all these subunits into the mature and functional cytochrome bc1 complex is therefore a complicated process which requires the participation of several chaperone proteins. It has been found that the assembly process of the mitochondrial bc1 complex proceeds through the formation of distinct sub-complexes in an ordered sequence. Most of these sub-complexes have been thoroughly characterized, and their molecular compositions have also been defined. This study critically analyses the results obtained so far and highlights new possible areas of investigation.
Keywords: chaperones; complex III; mitochondria; mitochondrial biogenesis; respiratory chain; respiratory complexes; respiratory sub-complexes; respiratory super-complexes; yeast strains.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

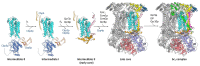

Similar articles
-
Biogenesis of the yeast cytochrome bc1 complex.Biochim Biophys Acta. 2009 Jan;1793(1):89-96. doi: 10.1016/j.bbamcr.2008.04.011. Epub 2008 May 3. Biochim Biophys Acta. 2009. PMID: 18501197 Review.
-
Further insights into the assembly of the yeast cytochrome bc1 complex based on analysis of single and double deletion mutants lacking supernumerary subunits and cytochrome b.Eur J Biochem. 2004 Mar;271(6):1209-18. doi: 10.1111/j.1432-1033.2004.04024.x. Eur J Biochem. 2004. PMID: 15009199
-
Respiratory complex III dysfunction in humans and the use of yeast as a model organism to study mitochondrial myopathy and associated diseases.Biochim Biophys Acta. 2013 Nov-Dec;1827(11-12):1346-61. doi: 10.1016/j.bbabio.2012.11.015. Epub 2012 Dec 5. Biochim Biophys Acta. 2013. PMID: 23220121 Review.
-
Evidence that the assembly of the yeast cytochrome bc1 complex involves the formation of a large core structure in the inner mitochondrial membrane.FEBS J. 2009 Apr;276(7):1900-14. doi: 10.1111/j.1742-4658.2009.06916.x. Epub 2009 Feb 19. FEBS J. 2009. PMID: 19236481 Free PMC article.
-
The dimerization of the yeast cytochrome bc1 complex is an early event and is independent of Rip1.Biochim Biophys Acta. 2015 May;1853(5):987-95. doi: 10.1016/j.bbamcr.2015.02.006. Epub 2015 Feb 13. Biochim Biophys Acta. 2015. PMID: 25683140
Cited by
-
Drug Drop Test: How to Quickly Identify Potential Therapeutic Compounds for Mitochondrial Diseases Using Yeast Saccharomyces cerevisiae.Int J Mol Sci. 2023 Jun 27;24(13):10696. doi: 10.3390/ijms241310696. Int J Mol Sci. 2023. PMID: 37445873 Free PMC article. Review.
-
Semiclassical Theory of Multistage Nonequilibrium Electron Transfer in Macromolecular Compounds in Polar Media with Several Relaxation Timescales.Int J Mol Sci. 2022 Dec 13;23(24):15793. doi: 10.3390/ijms232415793. Int J Mol Sci. 2022. PMID: 36555434 Free PMC article.
-
Coordination of cytochrome bc1 complex assembly at MICOS.EMBO Rep. 2025 Jan;26(2):353-384. doi: 10.1038/s44319-024-00336-x. Epub 2024 Dec 2. EMBO Rep. 2025. PMID: 39623166 Free PMC article.
References
-
- Trumpower B.L. The cytochrome bc1 complex. In: Lennarz V., Lane M.D., editors. Encyclopedia of Biological Chemistry. Elsevier Inc.; Amsterdam, The Netherlands: 2004. pp. 528–534.
-
- Brandt U., Yu L., Yu C.A., Trumpower B.L. The mitochondrial targeting presequence of the Rieske iron-sulfur protein is processed in a single step after insertion into the cytochrome bc1 complex in mammals and retained as a subunit in the complex. J. Biol. Chem. 1993;268:8387–8390. doi: 10.1016/S0021-9258(18)52883-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

