Assembly of the Multi-Subunit Cytochrome bc1 Complex in the Yeast Saccharomyces cerevisiae
- PMID: 36142449
- PMCID: PMC9502982
- DOI: 10.3390/ijms231810537
Assembly of the Multi-Subunit Cytochrome bc1 Complex in the Yeast Saccharomyces cerevisiae
Abstract
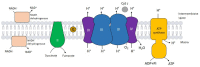
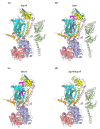
The cytochrome bc1 complex is an essential component of the mitochondrial respiratory chain of the yeast Saccharomyces cerevisiae. It is composed of ten protein subunits, three of them playing an important role in electron transfer and proton pumping across the inner mitochondrial membrane. Cytochrome b, the central component of this respiratory complex, is encoded by the mitochondrial genome, whereas all the other subunits are of nuclear origin. The assembly of all these subunits into the mature and functional cytochrome bc1 complex is therefore a complicated process which requires the participation of several chaperone proteins. It has been found that the assembly process of the mitochondrial bc1 complex proceeds through the formation of distinct sub-complexes in an ordered sequence. Most of these sub-complexes have been thoroughly characterized, and their molecular compositions have also been defined. This study critically analyses the results obtained so far and highlights new possible areas of investigation.
Keywords: chaperones; complex III; mitochondria; mitochondrial biogenesis; respiratory chain; respiratory complexes; respiratory sub-complexes; respiratory super-complexes; yeast strains.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

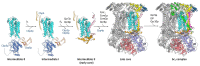

References
-
- Trumpower B.L. The cytochrome bc1 complex. In: Lennarz V., Lane M.D., editors. Encyclopedia of Biological Chemistry. Elsevier Inc.; Amsterdam, The Netherlands: 2004. pp. 528–534.
-
- Brandt U., Yu L., Yu C.A., Trumpower B.L. The mitochondrial targeting presequence of the Rieske iron-sulfur protein is processed in a single step after insertion into the cytochrome bc1 complex in mammals and retained as a subunit in the complex. J. Biol. Chem. 1993;268:8387–8390. doi: 10.1016/S0021-9258(18)52883-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

