Subfunctionalization of D27 Isomerase Genes in Saffron
- PMID: 36142456
- PMCID: PMC9504799
- DOI: 10.3390/ijms231810543
Subfunctionalization of D27 Isomerase Genes in Saffron
Abstract
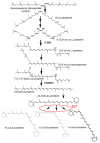
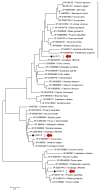
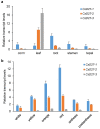
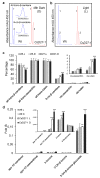
Chromoplasts and chloroplasts contain carotenoid pigments as all-trans- and cis-isomers, which function as accessory light-harvesting pigments, antioxidant and photoprotective agents, and precursors of signaling molecules and plant hormones. The carotenoid pathway involves the participation of different carotenoid isomerases. Among them, D27 is a β-carotene isomerase showing high specificity for the C9-C10 double bond catalyzing the interconversion of all-trans- into 9-cis-β-carotene, the precursor of strigolactones. We have identified one D27 (CsD27-1) and two D27-like (CsD27-2 and CsD27-3) genes in saffron, with CsD27-1 and CsD27-3, clearly differing in their expression patterns; specifically, CsD27-1 was mainly expressed in the undeveloped stigma and roots, where it is induced by Rhizobium colonization. On the contrary, CsD27-2 and CsD27-3 were mainly expressed in leaves, with a preferential expression of CsD27-3 in this tissue. In vivo assays show that CsD27-1 catalyzes the isomerization of all-trans- to 9-cis-β-carotene, and could be involved in the isomerization of zeaxanthin, while CsD27-3 catalyzes the isomerization of all-trans- to cis-ζ-carotene and all-trans- to cis-neurosporene. Our data show that CsD27-1 and CsD27-3 enzymes are both involved in carotenoid isomerization, with CsD27-1 being specific to chromoplast/amyloplast-containing tissue, and CsD27-3 more specific to chloroplast-containing tissues. Additionally, we show that CsD27-1 is co-expressed with CCD7 and CCD8 mycorrhized roots, whereas CsD27-3 is expressed at higher levels than CRTISO and Z-ISO and showed circadian regulation in leaves. Overall, our data extend the knowledge about carotenoid isomerization and their implications in several physiological and ecological processes.
Keywords: apocarotenoids; carotenoids; expression; isomerase activity; leaves; mycorrhiza; root; stigmas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27.Planta. 2016 Jun;243(6):1429-40. doi: 10.1007/s00425-016-2487-5. Epub 2016 Mar 5. Planta. 2016. PMID: 26945857
-
The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency.Plant Sci. 2018 Dec;277:33-42. doi: 10.1016/j.plantsci.2018.06.024. Epub 2018 Sep 11. Plant Sci. 2018. PMID: 30466598
-
D27-LIKE1 isomerase has a preference towards trans/cis and cis/cis conversions of carotenoids in Arabidopsis.Plant J. 2022 Dec;112(6):1377-1395. doi: 10.1111/tpj.16017. Epub 2022 Nov 21. Plant J. 2022. PMID: 36308414
-
D27-like carotenoid isomerases: at the crossroads of strigolactone and abscisic acid biosynthesis.J Exp Bot. 2024 Feb 12;75(4):1148-1158. doi: 10.1093/jxb/erad475. J Exp Bot. 2024. PMID: 38006582 Review.
-
From carotenoids to strigolactones.J Exp Bot. 2018 Apr 23;69(9):2189-2204. doi: 10.1093/jxb/erx476. J Exp Bot. 2018. PMID: 29253188 Review.
Cited by
-
Characterization of a β-carotene isomerase from the cyanobacterium Cyanobacteria aponinum.Philos Trans R Soc Lond B Biol Sci. 2024 Nov 18;379(1914):20230360. doi: 10.1098/rstb.2023.0360. Epub 2024 Sep 30. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39343012 Free PMC article.
-
Characterization of Carotenoid Cleavage Oxygenase Genes in Cerasus humilis and Functional Analysis of ChCCD1.Plants (Basel). 2023 May 26;12(11):2114. doi: 10.3390/plants12112114. Plants (Basel). 2023. PMID: 37299092 Free PMC article.
References
-
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D., Limon M.C., Melen-dez-Martinez A.J., Olmedilla-Alonso B., Palou A., et al. A global perspective on carote-noids: Metabolism, biotech-nology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. - DOI - PubMed
-
- Zhang Z., Hu Q., Liu Y., Cheng P., Cheng H., Liu W., Xing X., Guan Z., Fang W., Chen S., et al. Strigolac-tone represses the synthesis of melatonin, thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J. Pineal Res. 2019;67:e12582. doi: 10.1111/jpi.12582. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

