Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge
- PMID: 36142470
- PMCID: PMC9501188
- DOI: 10.3390/ijms231810558
Biophysical Characterization of LTX-315 Anticancer Peptide Interactions with Model Membrane Platforms: Effect of Membrane Surface Charge
Abstract
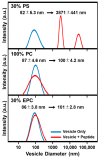
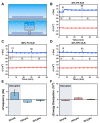
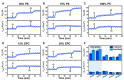
LTX-315 is a clinical-stage, anticancer peptide therapeutic that disrupts cancer cell membranes. Existing mechanistic knowledge about LTX-315 has been obtained from cell-based biological assays, and there is an outstanding need to directly characterize the corresponding membrane-peptide interactions from a biophysical perspective. Herein, we investigated the membrane-disruptive properties of the LTX-315 peptide using three cell-membrane-mimicking membrane platforms on solid supports, namely the supported lipid bilayer, intact vesicle adlayer, and tethered lipid bilayer, in combination with quartz crystal microbalance-dissipation (QCM-D) and electrochemical impedance spectroscopy (EIS) measurements. The results showed that the cationic LTX-315 peptide selectively disrupted negatively charged phospholipid membranes to a greater extent than zwitterionic or positively charged phospholipid membranes, whereby electrostatic interactions were the main factor to influence peptide attachment and membrane curvature was a secondary factor. Of note, the EIS measurements showed that the LTX-315 peptide extensively and irreversibly permeabilized negatively charged, tethered lipid bilayers that contained high phosphatidylserine lipid levels representative of the outer leaflet of cancer cell membranes, while circular dichroism (CD) spectroscopy experiments indicated that the LTX-315 peptide was structureless and the corresponding membrane-disruptive interactions did not involve peptide conformational changes. Dynamic light scattering (DLS) measurements further verified that the LTX-315 peptide selectively caused irreversible disruption of negatively charged lipid vesicles. Together, our findings demonstrate that the LTX-315 peptide preferentially disrupts negatively charged phospholipid membranes in an irreversible manner, which reinforces its potential as an emerging cancer immunotherapy and offers a biophysical framework to guide future peptide engineering efforts.
Keywords: LTX-315; anticancer peptide; electrochemical impedance spectroscopy; membrane-peptide interactions; oncolytic; peptide; quartz crystal microbalance-dissipation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes.Int J Mol Sci. 2022 Dec 29;24(1):581. doi: 10.3390/ijms24010581. Int J Mol Sci. 2022. PMID: 36614022 Free PMC article.
-
AH peptide-mediated formation of charged planar lipid bilayers.J Phys Chem B. 2014 Apr 3;118(13):3616-21. doi: 10.1021/jp411648s. Epub 2014 Mar 25. J Phys Chem B. 2014. PMID: 24628664
-
Ib-AMP4 insertion causes surface rearrangement in the phospholipid bilayer of biomembranes: Implications from quartz-crystal microbalance with dissipation.Biochim Biophys Acta Biomembr. 2018 Feb;1860(2):617-623. doi: 10.1016/j.bbamem.2017.10.025. Epub 2017 Oct 26. Biochim Biophys Acta Biomembr. 2018. PMID: 29106975
-
Nanoarchitectonics-based model membrane platforms for probing membrane-disruptive interactions of odd-chain antimicrobial lipids.Nano Converg. 2022 Nov 1;9(1):48. doi: 10.1186/s40580-022-00339-1. Nano Converg. 2022. PMID: 36318349 Free PMC article.
-
Engineering the bilayer: Emerging genetic tool kits for mechanistic lipid biology.Curr Opin Chem Biol. 2021 Dec;65:66-73. doi: 10.1016/j.cbpa.2021.05.013. Epub 2021 Jul 1. Curr Opin Chem Biol. 2021. PMID: 34218059 Free PMC article. Review.
Cited by
-
Antimicrobial peptides as potential therapy for gastrointestinal cancers.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2831-2841. doi: 10.1007/s00210-023-02536-z. Epub 2023 May 30. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37249612 Review.
-
Study of the Membrane Activity of the Synthetic Peptide ∆M3 Against Extended-Spectrum β-lactamase Escherichia coli Isolates.J Membr Biol. 2024 Apr;257(1-2):51-61. doi: 10.1007/s00232-024-00306-3. Epub 2024 Feb 5. J Membr Biol. 2024. PMID: 38315239 Free PMC article.
-
Understanding the Biophysical Interaction of LTX-315 with Tumoral Model Membranes.Int J Mol Sci. 2022 Dec 29;24(1):581. doi: 10.3390/ijms24010581. Int J Mol Sci. 2022. PMID: 36614022 Free PMC article.
-
Role of Anti-Cancer Peptides as Immunomodulatory Agents: Potential and Design Strategy.Pharmaceutics. 2022 Dec 1;14(12):2686. doi: 10.3390/pharmaceutics14122686. Pharmaceutics. 2022. PMID: 36559179 Free PMC article. Review.
-
Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study.Int J Mol Sci. 2023 May 25;24(11):9283. doi: 10.3390/ijms24119283. Int J Mol Sci. 2023. PMID: 37298235 Free PMC article.
References
-
- Gabernet G., Müller A.T., Hiss J.A., Schneider G. Membranolytic anticancer peptides. MedChemComm. 2016;7:2232–2245. doi: 10.1039/C6MD00376A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

