Recombinant Reg3α Prevents Islet β-Cell Apoptosis and Promotes β-Cell Regeneration
- PMID: 36142497
- PMCID: PMC9504149
- DOI: 10.3390/ijms231810584
Recombinant Reg3α Prevents Islet β-Cell Apoptosis and Promotes β-Cell Regeneration
Abstract
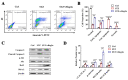
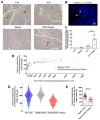
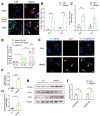
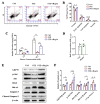
Progressive loss and dysfunction of islet β-cells has not yet been solved in the treatment of diabetes. Regenerating protein (Reg) has been identified as a trophic factor which is demonstrated to be associated with pancreatic tissue regeneration. We previously produced recombinant Reg3α protein (rReg3α) and proved that it protects against acute pancreatitis in mice. Whether rReg3α protects islet β-cells in diabetes has been elusive. In the present study, rReg3α stimulated MIN6 cell proliferation and resisted STZ-caused cell death. The protective effect of rReg3α was also found in mouse primary islets. In BALB/c mice, rReg3α administration largely alleviated STZ-induced diabetes by the preservation of β-cell mass. The protective mechanism could be attributed to Akt/Bcl-2/-xL activation and GRP78 upregulation. Scattered insulin-expressing cells and clusters with small size, low insulin density, and exocrine distribution were observed and considered to be neogenic. In isolated acinar cells with wheat germ agglutinin (WGA) labeling, rReg3α treatment generated insulin-producing cells through Stat3/Ngn3 signaling, but these cells were not fully functional in response to glucose stimulation. Our results demonstrated that rReg3α resists STZ-induced β-cell death and promotes β-cell regeneration. rReg3α could serve as a potential drug for β-cell maintenance in anti-diabetic treatment.
Keywords: GRP78; diabetes; regenerating protein; the islets; β-cell regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Recombinant Reg3α protein protects against experimental acute pancreatitis in mice.Mol Cell Endocrinol. 2016 Feb 15;422:150-159. doi: 10.1016/j.mce.2015.12.002. Epub 2015 Dec 9. Mol Cell Endocrinol. 2016. PMID: 26683606
-
Recombinant Reg3β protein protects against streptozotocin-induced β-cell damage and diabetes.Sci Rep. 2016 Oct 21;6:35640. doi: 10.1038/srep35640. Sci Rep. 2016. PMID: 27767186 Free PMC article.
-
Dimorphic autoantigenic and protective effects of Reg2 peptide in the treatment of diabetic β-cell loss.Diabetes Obes Metab. 2019 May;21(5):1209-1222. doi: 10.1111/dom.13644. Epub 2019 Mar 1. Diabetes Obes Metab. 2019. PMID: 30690849
-
The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells.J Hepatobiliary Pancreat Surg. 1999;6(3):254-62. doi: 10.1007/s005340050115. J Hepatobiliary Pancreat Surg. 1999. PMID: 10526060 Review.
-
The many lives of Myc in the pancreatic β-cell.J Biol Chem. 2021 Jan-Jun;296:100122. doi: 10.1074/jbc.REV120.011149. Epub 2020 Dec 2. J Biol Chem. 2021. PMID: 33239359 Free PMC article. Review.
Cited by
-
Dysfunctional β-cell autophagy induces β-cell stress and enhances islet immunogenicity.Front Immunol. 2025 Jan 29;16:1504583. doi: 10.3389/fimmu.2025.1504583. eCollection 2025. Front Immunol. 2025. PMID: 39944686 Free PMC article.
-
Genetics of constant and severe pain in the NAPS2 cohort of recurrent acute and chronic pancreatitis patients.J Pain. 2025 Feb;27:104754. doi: 10.1016/j.jpain.2024.104754. Epub 2024 Dec 12. J Pain. 2025. PMID: 39674387 Free PMC article.
-
Talin-1 inhibits Smurf1-mediated Stat3 degradation to modulate β-cell proliferation and mass in mice.Cell Death Dis. 2023 Oct 31;14(10):709. doi: 10.1038/s41419-023-06235-8. Cell Death Dis. 2023. PMID: 37903776 Free PMC article.
-
Controversial Roles of Regenerating Family Proteins in Tissue Repair and Tumor Development.Biomedicines. 2024 Dec 26;13(1):24. doi: 10.3390/biomedicines13010024. Biomedicines. 2024. PMID: 39857608 Free PMC article. Review.
-
Identification of eight genomic protective alleles for mitochondrial diabetes by Kinship-graph convolutional network.J Diabetes Investig. 2024 Jan;15(1):52-62. doi: 10.1111/jdi.14125. Epub 2023 Dec 29. J Diabetes Investig. 2024. PMID: 38157301 Free PMC article.
References
-
- Retnakaran R., Rn A.E., Ye C., Harris S.B., Reichert S.M., McInnes N., Gerstein H.C., Thorpe K.E., Kramer C.K., Zinman B., et al. Short-term intensive insulin as induction and maintenance therapy for the preservation of beta-cell function in early type 2 diabetes (RESET-IT Main): A 2-year randomized controlled trial. Diabetes, Obes. Metab. 2021;23:1926–1935. doi: 10.1111/dom.14421. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

