Disclosing the Biocide Activity of α-Ag2-2 xCu x WO4 (0 ≤ x ≤ 0.16) Solid Solutions
- PMID: 36142511
- PMCID: PMC9504239
- DOI: 10.3390/ijms231810589
Disclosing the Biocide Activity of α-Ag2-2 xCu x WO4 (0 ≤ x ≤ 0.16) Solid Solutions
Abstract
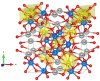
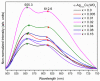
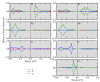
In this work, α-Ag2-2xCuxWO4 (0 ≤ x ≤ 0.16) solid solutions with enhanced antibacterial (against methicillin-resistant Staphylococcus aureus) and antifungal (against Candida albicans) activities are reported. A plethora of techniques (X-ray diffraction with Rietveld refinements, inductively coupled plasma atomic emission spectrometry, micro-Raman spectroscopy, attenuated total reflectance-Fourier transform infrared spectroscopy, field emission scanning electron microscopy, ultraviolet-visible spectroscopy, photoluminescence emissions, and X-ray photoelectron spectroscopy) were employed to characterize the as-synthetized samples and determine the local coordination geometry of Cu2+ cations at the orthorhombic lattice. To find a correlation between morphology and biocide activity, the experimental results were sustained by first-principles calculations at the density functional theory level to decipher the cluster coordinations and electronic properties of the exposed surfaces. Based on the analysis of the under-coordinated Ag and Cu clusters at the (010) and (101) exposed surfaces, we propose a mechanism to explain the biocide activity of these solid solutions.
Keywords: DFT study; biocide activity; morphology; α-Ag2−2xCuxWO4 solid solutions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Jovanovic D.J., Validzic I.L., Mitric M., Nedeljkovic J.M. Synthesis and structural characterization of nano-sized copper tungstate particles. Acta Chim. Slov. 2012;59:70. - PubMed
-
- Jacomaci N., Junior E.S., Oliveira F.M.B.d., Longo E., Zaghete M.A. Dielectric behavior of α-Ag2WO4 and its huge dielectric loss tangent. Mater. Res. 2019;22:1. doi: 10.1590/1980-5373-mr-2019-0058. - DOI
-
- Tang J.W., Ye J.H. Correlation of crystal structures and electronic structures and photocatalytic properties of the W-containing oxides. J. Mater. Chem. 2005;15:4246. doi: 10.1039/b504818d. - DOI
-
- Sreedevi A., Priyanka K.P., Babitha K.K., Sabu N.A., Anu T.S., Varghese T. Chemical synthesis, structural characterization and optical properties of nanophase α-Ag2WO4. Indian J. Phys. 2015;89:889. doi: 10.1007/s12648-015-0664-1. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

