Sodium Accumulation in Infected Cells and Ion Transporters Mistargeting in Nodules of Medicago truncatula: Two Ugly Items That Hinder Coping with Salt Stress Effects
- PMID: 36142539
- PMCID: PMC9505113
- DOI: 10.3390/ijms231810618
Sodium Accumulation in Infected Cells and Ion Transporters Mistargeting in Nodules of Medicago truncatula: Two Ugly Items That Hinder Coping with Salt Stress Effects
Abstract
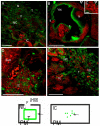
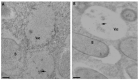
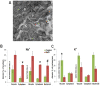
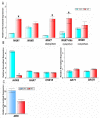
The maintenance of intracellular nitrogen-fixing bacteria causes changes in proteins' location and in gene expression that may be detrimental to the host cell fitness. We hypothesized that the nodule's high vulnerability toward salt stress might be due to alterations in mechanisms involved in the exclusion of Na+ from the host cytoplasm. Confocal and electron microscopy immunolocalization analyses of Na+/K+ exchangers in the root nodule showed the plasma membrane (MtNHX7) and endosome/tonoplast (MtNHX6) signal in non-infected cells; however, in mature infected cells the proteins were depleted from their target membranes and expelled to vacuoles. This mistargeting suggests partial loss of the exchanger's functionality in these cells. In the mature part of the nodule 7 of the 20 genes encoding ion transporters, channels, and Na+/K+ exchangers were either not expressed or substantially downregulated. In nodules from plants subjected to salt treatments, low temperature-scanning electron microscopy and X-ray microanalysis revealed the accumulation of 5-6 times more Na+ per infected cell versus non-infected one. Hence, the infected cells' inability to withstand the salt may be the integral result of preexisting defects in the localization of proteins involved in Na+ exclusion and the reduced expression of key genes of ion homeostasis, resulting in premature senescence and termination of symbiosis.
Keywords: Medicago truncatula; NHX Na+/K+ exchangers; ion transport; potassium; root nodule; salt stress; sodium; symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Roth L.E., Jeon K.W., Stacey G. Homology in endosymbiotic systems: The term ‘symbiosome’. In: Palacios R.A.V.D.P.S., editor. Molecular Genetics of Plant-Microbe Interactions. APS Press; St-Paul, MN, USA: 1988. pp. 220–225.
-
- Gavrin A., Jansen V., Ivanov S., Bisseling T., Fedorova E. ARP2/3-Mediated Actin Nucleation Associated with Symbiosome Membrane Is Essential for the Development of Symbiosomes in Infected Cells of Medicago truncatula Root Nodules. Mol. Plant-Microbe Interact. 2015;28:605–614. doi: 10.1094/MPMI-12-14-0402-R. - DOI - PubMed
-
- Chakraborty S., Driscoll H.E., Abrahante J.E., Zhang F., Fisher R.F., Harris J.M. Salt Stress Enhances Early Symbiotic Gene Expression in Medicago truncatula and Induces a Stress-Specific Set of Rhizobium-Responsive Genes. Mol. Plant-Microbe Interact. 2021;34:904–921. doi: 10.1094/MPMI-01-21-0019-R. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- grant S2009/AMB-151/Consejería de Economía, Empleo y Competitividad Comunidad de Madrid
- grant AGL2013-40758-R/MINECO
- No. АААА-А19-119041690042-7 and No. 122042700044-6/Ministry of Science and Higher Education of the Russian Federation
- AGL2017-88381-R and PID2021-11253710B-100to JJP and MML/AEI/FEDER-UE
- 19-04-00570 to EEF/RFBR (Russian Foundation for Basic Research)
LinkOut - more resources
Full Text Sources

