CD73-Adenosinergic Axis Mediates the Protective Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells on Ischemic Renal Damage in a Rat Model of Donation after Circulatory Death
- PMID: 36142593
- PMCID: PMC9501320
- DOI: 10.3390/ijms231810681
CD73-Adenosinergic Axis Mediates the Protective Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells on Ischemic Renal Damage in a Rat Model of Donation after Circulatory Death
Abstract
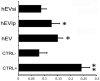
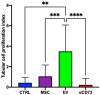
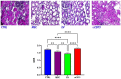
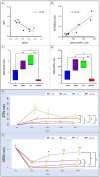
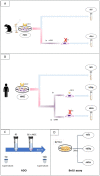
We propose a new organ-conditioning strategy based on mesenchymal stromal cell (MSCs)/extracellular vesicle (EVs) delivery during hypothermic perfusion. MSCs/EVs marker CD73 is present on renal proximal tubular cells, and it protects against renal ischemia-reperfusion injury by converting adenosine monophosphate into adenosine (ADO). In this study, after checking if CD73-silenced EVs (EVsi) would impact in vitro tubular-cell proliferation, we perfused kidneys of a rat model of donation after circulatory death, with Belzer solution (BS) alone, BS supplemented with MSCs, EVs, or EVsi. The ADO and ATP levels were measured in the effluents and tissues. Global renal ischemic damage score (GRS), and tubular cell proliferation index (IPT) were evaluated in the tissue. EVsi did not induce cell proliferation in vitro. Ex vivo kidneys perfused with BS or BS + EVsi showed the worst GRS and higher effluent ADO levels than the MSC- and EV-perfused kidneys. In the EV-perfused kidneys, the tissue and effluent ATP levels and IPT were the highest, but not if CD73 was silenced. Tissue ATP content was positively correlated with tissue ADO content and negatively correlated with effluent ADO level in all groups. In conclusion, kidney conditioning with EVs protects against ischemic damage by activating the CD73/ADO system.
Keywords: CD73; adenosine; extracellular vesicles; ischemia-reperfusion injury; kidney transplantation; mesenchymal stromal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gregorini M., Corradetti V., Rocca C., Pattonieri E.F., Valsania T., Milanesi S., Serpieri N., Bedino G., Esposito P., Libetta C., et al. Mesenchymal Stromal Cells Prevent Renal Fibrosis in a Rat Model of Unilateral Ureteral Obstruction by Suppressing the Renin-Angiotensin System via HuR. PLoS ONE. 2016;11:e0148542. doi: 10.1371/journal.pone.0148542. - DOI - PMC - PubMed
-
- Gregorini M., Bosio F., Rocca C., Corradetti V., Valsania T., Pattonieri E.F., Esposito P., Bedino G., Collesi C., Libetta C., et al. Mesenchymal Stromal Cells Reset the Scatter Factor System and Cytokine Network in Experimental Kidney Transplantation. BMC Immunol. 2014;15:44. doi: 10.1186/s12865-014-0044-1. - DOI - PMC - PubMed
-
- Rampino T., Gregorini M., Bedino G., Piotti G., Gabanti E., Ibatici A., Sessarego N., Piacenza C., Balenzano C.T., Esposito P., et al. Mesenchymal Stromal Cells Improve Renal Injury in Anti-Thy 1 Nephritis by Modulating Inflammatory Cytokines and Scatter Factors. Clin. Sci. Lond. Engl. 1979. 2011;120:25–36. doi: 10.1042/CS20100147. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

