Novel Anti-Neuroinflammatory Properties of a Thiosemicarbazone-Pyridylhydrazone Copper(II) Complex
- PMID: 36142627
- PMCID: PMC9505367
- DOI: 10.3390/ijms231810722
Novel Anti-Neuroinflammatory Properties of a Thiosemicarbazone-Pyridylhydrazone Copper(II) Complex
Abstract
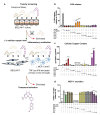
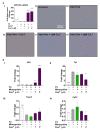
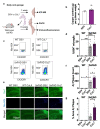
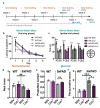
Neuroinflammation has a major role in several brain disorders including Alzheimer's disease (AD), yet at present there are no effective anti-neuroinflammatory therapeutics available. Copper(II) complexes of bis(thiosemicarbazones) (CuII(gtsm) and CuII(atsm)) have broad therapeutic actions in preclinical models of neurodegeneration, with CuII(atsm) demonstrating beneficial outcomes on neuroinflammatory markers in vitro and in vivo. These findings suggest that copper(II) complexes could be harnessed as a new approach to modulate immune function in neurodegenerative diseases. In this study, we examined the anti-neuroinflammatory action of several low-molecular-weight, charge-neutral and lipophilic copper(II) complexes. Our analysis revealed that one compound, a thiosemicarbazone-pyridylhydrazone copper(II) complex (CuL5), delivered copper into cells in vitro and increased the concentration of copper in the brain in vivo. In a primary murine microglia culture, CuL5 was shown to decrease secretion of pro-inflammatory cytokine macrophage chemoattractant protein 1 (MCP-1) and expression of tumor necrosis factor alpha (Tnf), increase expression of metallothionein (Mt1), and modulate expression of Alzheimer's disease-associated risk genes, Trem2 and Cd33. CuL5 also improved the phagocytic function of microglia in vitro. In 5xFAD model AD mice, treatment with CuL5 led to an improved performance in a spatial working memory test, while, interestingly, increased accumulation of amyloid plaques in treated mice. These findings demonstrate that CuL5 can induce anti-neuroinflammatory effects in vitro and provide selective benefit in vivo. The outcomes provide further support for the development of copper-based compounds to modulate neuroinflammation in brain diseases.
Keywords: Alzheimer’s disease; copper; inflammation; microglia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Cribbs D.H., Berchtold N.C., Perreau V., Coleman P.D., Rogers J., Tenner A.J., Cotman C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflamm. 2012;9:179. doi: 10.1186/1742-2094-9-179. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

