Quantitative Analysis of Plant Cytosolic Calcium Signals in Response to Water Activated by Low-Power Non-Thermal Plasma
- PMID: 36142664
- PMCID: PMC9506352
- DOI: 10.3390/ijms231810752
Quantitative Analysis of Plant Cytosolic Calcium Signals in Response to Water Activated by Low-Power Non-Thermal Plasma
Abstract
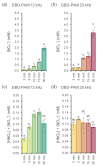
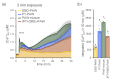
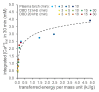
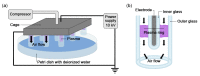
Non-thermal plasma technology is increasingly being applied in the plant biology field. Despite the variety of beneficial effects of plasma-activated water (PAW) on plants, information about the mechanisms of PAW sensing by plants is still limited. In this study, in order to link PAW perception to the positive downstream responses of plants, transgenic Arabidopsis thaliana seedlings expressing the Ca2+-sensitive photoprotein aequorin in the cytosol were challenged with water activated by low-power non-thermal plasma generated by a dielectric barrier discharge (DBD) source. PAW sensing by plants resulted in the occurrence of cytosolic Ca2+ signals, whose kinetic parameters were found to strictly depend on the operational conditions of the plasma device and thus on the corresponding mixture of chemical species contained in the PAW. In particular, we highlighted the effect on the intracellular Ca2+ signals of low doses of DBD-PAW chemicals and also presented the effects of consecutive plant treatments. The results were discussed in terms of the possibility of using PAW-triggered Ca2+ signatures as benchmarks to accurately modulate the chemical composition of PAW in order to induce environmental stress resilience in plants, thus paving the way for further applications in agriculture.
Keywords: Arabidopsis thaliana; aequorin; chemical analyses; cytosolic Ca2+ transients; dielectric barrier discharge; plant calcium signalling; plasma activated water; plasma torch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

