Increased ACS Enzyme Dosage Causes Initiation of Climacteric Ethylene Production in Tomato
- PMID: 36142701
- PMCID: PMC9501751
- DOI: 10.3390/ijms231810788
Increased ACS Enzyme Dosage Causes Initiation of Climacteric Ethylene Production in Tomato
Abstract
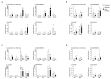
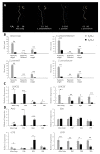
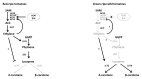
Fruits of wild tomato species show different ethylene-dependent ripening characteristics, such as variations in fruit color and whether they exhibit a climacteric or nonclimacteric ripening transition. 1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) are key enzymes in the ethylene biosynthetic pathway encoded by multigene families. Gene duplication is a primary driver of plant diversification and angiosperm evolution. Here, interspecific variations in the molecular regulation of ethylene biosynthesis and perception during fruit ripening in domesticated and wild tomatoes were investigated. Results showed that the activated ACS genes were increased in number in red-ripe tomato fruits than in green-ripe tomato fruits; therefore, elevated dosage of ACS enzyme promoted ripening ethylene production. Results showed that the expression of three ACS isogenes ACS1A, ACS2, and ACS4, which are involved in autocatalytic ethylene production, was higher in red-ripe tomato fruits than in green-ripe tomato fruits. Elevated ACS enzyme dosage promoted ethylene production, which corresponded to the climacteric response of red-ripe tomato fruits. The data suggest that autoinhibitory ethylene production is common to all tomato species, while autocatalytic ethylene production is specific to red-ripe species. The essential regulators Non-ripening (NOR) and Ripening-Inhibitor (RIN) have experienced gene activation and overlapped with increasing ACS enzyme dosage. These complex levels of transcript regulation link higher ethylene production with spatiotemporal modulation of gene expression in red-ripe tomato species. Taken together, this study shows that bursts in ethylene production that accompany fruit color changes in red-ripe tomatoes are likely to be an evolutionary adaptation for seed dispersal.
Keywords: climacteric; ethylene biosynthesis; fruit ripening; gene duplication; wild tomato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
ACS4 exerts a pivotal role in ethylene biosynthesis during the ripening of tomato fruits in comparison to ACS2.Plant J. 2025 Mar;121(5):e70043. doi: 10.1111/tpj.70043. Plant J. 2025. PMID: 40040541
-
The regulation mode of RIN transcription factor involved in ethylene biosynthesis in tomato fruit.J Sci Food Agric. 2011 Aug 15;91(10):1822-8. doi: 10.1002/jsfa.4390. Epub 2011 Apr 26. J Sci Food Agric. 2011. PMID: 21520447
-
Characterisation of ethylene pathway components in non-climacteric capsicum.BMC Plant Biol. 2013 Nov 28;13:191. doi: 10.1186/1471-2229-13-191. BMC Plant Biol. 2013. PMID: 24286334 Free PMC article.
-
Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening.J Exp Bot. 2002 Oct;53(377):2039-55. doi: 10.1093/jxb/erf072. J Exp Bot. 2002. PMID: 12324528 Review.
-
Ethylene receptors and related proteins in climacteric and non-climacteric fruits.Plant Sci. 2018 Nov;276:63-72. doi: 10.1016/j.plantsci.2018.07.012. Epub 2018 Aug 10. Plant Sci. 2018. PMID: 30348329 Review.
Cited by
-
Genome-Wide Identification and Expression Analysis of 1-Aminocyclopropane-1-Carboxylate Synthase (ACS) Gene Family in Myrica rubra.Int J Mol Sci. 2025 May 10;26(10):4580. doi: 10.3390/ijms26104580. Int J Mol Sci. 2025. PMID: 40429726 Free PMC article.
-
The triggering mechanism for predominant hormonal signal production in fleshy fruit ripening.Mol Hortic. 2025 Jun 6;5(1):35. doi: 10.1186/s43897-025-00155-1. Mol Hortic. 2025. PMID: 40474307 Free PMC article. Review.
-
Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum).Int J Mol Sci. 2024 Jun 12;25(12):6493. doi: 10.3390/ijms25126493. Int J Mol Sci. 2024. PMID: 38928199 Free PMC article.
-
ChIP-Seq Analysis of SlAREB1 Downstream Regulatory Network during Tomato Ripening.Foods. 2023 Jun 13;12(12):2357. doi: 10.3390/foods12122357. Foods. 2023. PMID: 37372568 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

