Medical Advances in Hepatitis D Therapy: Molecular Targets
- PMID: 36142728
- PMCID: PMC9506394
- DOI: 10.3390/ijms231810817
Medical Advances in Hepatitis D Therapy: Molecular Targets
Abstract
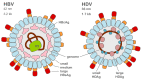
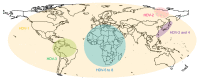
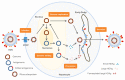
An approximate number of 250 million people worldwide are chronically infected with hepatitis B virus, making them susceptible to a coinfection with hepatitis D virus. The superinfection causes the most severe form of a viral hepatitis and thus drastically worsens the course of the disease. Until recently, the only available therapy consisted of interferon-α, only eligible for a minority of patients. In July 2020, the EMA granted Hepcludex conditional marketing authorization throughout the European Union. This first-in-class entry inhibitor offers the promise to prevent the spread in order to gain control and eventually participate in curing hepatitis B and D. Hepcludex is an example of how understanding the viral lifecycle can give rise to new therapy options. Sodium taurocholate co-transporting polypeptide, the virus receptor and the target of Hepcludex, and other targets of hepatitis D therapy currently researched are reviewed in this work. Farnesyltransferase inhibitors such as Lonafarnib, targeting another essential molecule in the HDV life cycle, represent a promising target for hepatitis D therapy. Farnesyltransferase attaches a farnesyl (isoprenyl) group to proteins carrying a C-terminal Ca1a2X (C: cysteine, a: aliphatic amino acid, X: C-terminal amino acid) motif like the large hepatitis D virus antigen. This modification enables the interaction of the HBV/HDV particle and the virus envelope proteins. Lonafarnib, which prevents this envelopment, has been tested in clinical trials. Targeting the lifecycle of the hepatitis B virus needs to be considered in hepatitis D therapy in order to cure a patient from both coexisting infections. Nucleic acid polymers target the hepatitis B lifecycle in a manner that is not yet understood. Understanding the possible targets of the hepatitis D virus therapy is inevitable for the improvement and development of a sufficient therapy that HDV patients are desperately in need of.
Keywords: Hepcludex; antivirals; farnesyl transferase; hepatitis B; hepatitis D; viral entry mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide.J Virol. 2014 Mar;88(6):3273-84. doi: 10.1128/JVI.03478-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390325 Free PMC article.
-
Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial.Lancet Gastroenterol Hepatol. 2017 Dec;2(12):877-889. doi: 10.1016/S2468-1253(17)30288-1. Epub 2017 Sep 28. Lancet Gastroenterol Hepatol. 2017. PMID: 28964701 Clinical Trial.
-
Bona fide receptor for hepatitis B and D viral infections: Mechanism, research models and molecular drug targets.Emerg Microbes Infect. 2018 Jul 26;7(1):134. doi: 10.1038/s41426-018-0137-7. Emerg Microbes Infect. 2018. PMID: 30050063 Free PMC article. Review.
-
Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide.PLoS Pathog. 2015 Apr 22;11(4):e1004840. doi: 10.1371/journal.ppat.1004840. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25902143 Free PMC article.
-
Hepatitis D virus: Improving virological knowledge to develop new treatments.Antiviral Res. 2023 Jan;209:105461. doi: 10.1016/j.antiviral.2022.105461. Epub 2022 Nov 14. Antiviral Res. 2023. PMID: 36396025 Review.
Cited by
-
Interferon-Free Regimens and Direct-Acting Antiviral Agents for Delta Hepatitis: Are We There Yet?Curr Issues Mol Biol. 2023 Sep 28;45(10):7878-7890. doi: 10.3390/cimb45100498. Curr Issues Mol Biol. 2023. PMID: 37886941 Free PMC article. Review.
-
Cytotoxic CD4+ T cells in chronic viral infections and cancer.Front Immunol. 2023 Oct 25;14:1271236. doi: 10.3389/fimmu.2023.1271236. eCollection 2023. Front Immunol. 2023. PMID: 37965314 Free PMC article. Review.
-
Current Treatment Regimens and Promising Molecular Therapies for Chronic Hepatobiliary Diseases.Biomolecules. 2025 Jan 14;15(1):121. doi: 10.3390/biom15010121. Biomolecules. 2025. PMID: 39858515 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

