Use of a Molecular Switch Probe to Activate or Inhibit GIRK1 Heteromers In Silico Reveals a Novel Gating Mechanism
- PMID: 36142730
- PMCID: PMC9502415
- DOI: 10.3390/ijms231810820
Use of a Molecular Switch Probe to Activate or Inhibit GIRK1 Heteromers In Silico Reveals a Novel Gating Mechanism
Abstract
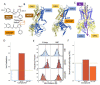
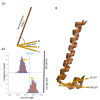
G protein-gated inwardly rectifying K+ (GIRK) channels form highly active heterotetramers in the body, such as in neurons (GIRK1/GIRK2 or GIRK1/2) and heart (GIRK1/GIRK4 or GIRK1/4). Based on three-dimensional atomic resolution structures for GIRK2 homotetramers, we built heterotetrameric GIRK1/2 and GIRK1/4 models in a lipid bilayer environment. By employing a urea-based activator ML297 and its molecular switch, the inhibitor GAT1587, we captured channel gating transitions and K+ ion permeation in sub-microsecond molecular dynamics (MD) simulations. This allowed us to monitor the dynamics of the two channel gates (one transmembrane and one cytosolic) as well as their control by the required phosphatidylinositol bis 4-5-phosphate (PIP2). By comparing differences in the two trajectories, we identify three hydrophobic residues in the transmembrane domain 1 (TM1) of GIRK1, namely, F87, Y91, and W95, which form a hydrophobic wire induced by ML297 and de-induced by GAT1587 to orchestrate channel gating. This includes bending of the TM2 and alignment of a dipole of two acidic GIRK1 residues (E141 and D173) in the permeation pathway to facilitate K+ ion conduction. Moreover, the TM movements drive the movement of the Slide Helix relative to TM1 to adjust interactions of the CD-loop that controls the gating of the cytosolic gate. The simulations reveal that a key basic residue that coordinates PIP2 to stabilize the pre-open and open states of the transmembrane gate flips in the inhibited state to form a direct salt-bridge interaction with the cytosolic gate and destabilize its open state.
Keywords: GIRK1 heterotetramer; MD simulations; ML297; PIP2; TM1 hydrophobic wire.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

