Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity
- PMID: 36142735
- PMCID: PMC9505659
- DOI: 10.3390/ijms231810825
Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity
Abstract
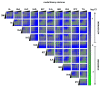
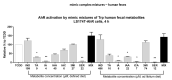
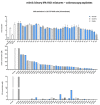
Aryl hydrocarbon receptor (AHR) plays pivotal roles in intestinal physiology and pathophysiology. Intestinal AHR is activated by numerous dietary, endogenous, and microbial ligands. Whereas the effects of individual compounds on AHR are mostly known, the effects of real physiological mixtures occurring in the intestine have not been studied. Using reporter gene assays and RT-PCR, we evaluated the combinatorial effects (3520 combinations) of 11 microbial catabolites of tryptophan (MICTs) on AHR. We robustly (n = 30) determined the potencies and relative efficacies of single MICTs. Synergistic effects of MICT binary mixtures were observed between low- or medium-efficacy agonists, in particular for combinations of indole-3-propionate and indole-3-lactate. Combinations comprising highly efficacious agonists such as indole-3-pyruvate displayed rather antagonist effects, caused by saturation of the assay response. These synergistic effects were confirmed by RT-PCR as CYP1A1 mRNA expression. We also tested mimic multicomponent and binary mixtures of MICTs, prepared based on the metabolomic analyses of human feces and colonoscopy aspirates, respectively. In this case, AHR responsiveness did not correlate with type of diet or health status, and the indole concentrations in the mixtures were determinative of gross AHR activity. Future systematic research on the synergistic activation of AHR by microbial metabolites and other ligands is needed.
Keywords: aryl hydrocarbon receptor; indole derivatives; microbiome; mimic mixtures; tryptophan metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Gut Microbial Catabolites of Tryptophan Are Ligands and Agonists of the Aryl Hydrocarbon Receptor: A Detailed Characterization.Int J Mol Sci. 2020 Apr 9;21(7):2614. doi: 10.3390/ijms21072614. Int J Mol Sci. 2020. PMID: 32283770 Free PMC article.
-
Serotonin Modulates AhR Activation by Interfering with CYP1A1-Mediated Clearance of AhR Ligands.Cell Physiol Biochem. 2020 Feb 5;54(1):126-141. doi: 10.33594/000000209. Cell Physiol Biochem. 2020. PMID: 32017483 Free PMC article.
-
Methylindoles and Methoxyindoles are Agonists and Antagonists of Human Aryl Hydrocarbon Receptor.Mol Pharmacol. 2018 Jun;93(6):631-644. doi: 10.1124/mol.118.112151. Epub 2018 Apr 6. Mol Pharmacol. 2018. PMID: 29626056 Free PMC article.
-
Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation.Drug Metab Dispos. 2015 Oct;43(10):1522-35. doi: 10.1124/dmd.115.064246. Epub 2015 Jun 3. Drug Metab Dispos. 2015. PMID: 26041783 Free PMC article. Review.
-
Indole scaffolds as a promising class of the aryl hydrocarbon receptor ligands.Eur J Med Chem. 2021 Apr 5;215:113231. doi: 10.1016/j.ejmech.2021.113231. Epub 2021 Feb 4. Eur J Med Chem. 2021. PMID: 33582577 Review.
Cited by
-
Bifidobacterium breve-derived indole-3-lactic acid ameliorates colitis-associated tumorigenesis by directing the differentiation of immature colonic macrophages.Theranostics. 2024 Apr 22;14(7):2719-2735. doi: 10.7150/thno.92350. eCollection 2024. Theranostics. 2024. PMID: 38773969 Free PMC article.
-
Indole-3-pyruvic acid alleviates rheumatoid arthritis via the aryl hydrocarbon receptor pathway.Ann Transl Med. 2023 Mar 15;11(5):213. doi: 10.21037/atm-23-1074. Ann Transl Med. 2023. PMID: 37007545 Free PMC article.
-
Jasmone Is a Ligand-Selective Allosteric Antagonist of Aryl Hydrocarbon Receptor (AhR).Int J Mol Sci. 2023 Oct 27;24(21):15655. doi: 10.3390/ijms242115655. Int J Mol Sci. 2023. PMID: 37958638 Free PMC article.
-
Maternal PBDE exposure disrupts gut microbiome and promotes hepatic proinflammatory signaling in humanized PXR-transgenic mouse offspring over time.Toxicol Sci. 2023 Jul 28;194(2):209-225. doi: 10.1093/toxsci/kfad056. Toxicol Sci. 2023. PMID: 37267213 Free PMC article.
-
Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy.Metabolites. 2024 Aug 11;14(8):444. doi: 10.3390/metabo14080444. Metabolites. 2024. PMID: 39195540 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 20-00449S/Czech Science Foundation
- CZ-OPENSCREEN (LM2018130)/Czech Ministry of Education, Youth and Sports
- EATRIS-CZ (LM2018133)/Czech Ministry of Education, Youth and Sports
- ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868)/the European Regional Development Fund
- (Programme EXCELES, ID Project No. LX22NPO5102)/National Institute for Cancer Research
LinkOut - more resources
Full Text Sources

