Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer's Disease from Other Neurological Disorders
- PMID: 36142742
- PMCID: PMC9501545
- DOI: 10.3390/ijms231810831
Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer's Disease from Other Neurological Disorders
Abstract
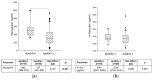
Recently, the synaptic proteins neurogranin (Ng) and α-synuclein (α-Syn) have attracted scientific interest as potential biomarkers for synaptic dysfunction in neurodegenerative diseases. In this study, we measured the CSF Ng and α-Syn concentrations in patients affected by AD (n = 69), non-AD neurodegenerative disorders (n-AD = 50) and non-degenerative disorders (n-ND, n = 98). The concentrations of CSF Ng and α-Syn were significantly higher in AD than in n-AD and n-ND. Moreover, the Aβ42/Ng and Aβ42/α-Syn ratios showed statistically significant differences between groups and discriminated AD patients from n-AD patients, better than Ng or α-Syn alone. Regression analyses showed an association of higher Ng concentrations with MMSE < 24, pathological Aβ 42/40 ratios, pTau, tTau and the ApoEε4 genotype. Aβ 42/Ng was associated with MMSE < 24, an AD-related FDG-PET pattern, the ApoEε4 genotype, pathological Aβ 42 levels and Aβ 42/40 ratios, pTau, and tTau. Moreover, APO-Eε4 carriers showed higher Ng concentrations than non-carriers. Our results support the idea that the Aβ 42/Ng ratio is a reliable index of synaptic dysfunction/degeneration able to discriminate AD from other neurological conditions.
Keywords: Alzheimer’s disease; biomarkers; neurogranin; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Agnello L., Piccoli T., Vidali M., Cuffaro L., Lo Sasso B., Iacolino G., Giglio V.R., Lupo F., Alongi P., Bivona G., et al. Diagnostic accuracy of cerebrospinal fluid biomarkers measured by chemiluminescent enzyme immunoassay for Alzheimer disease diagnosis. Scand. J. Clin. Lab. Investig. 2020;80:313–317. doi: 10.1080/00365513.2020.1740939. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

