Engineering Nanofiber Scaffolds with Biomimetic Cues for Differentiation of Skin-Derived Neural Crest-like Stem Cells to Schwann Cells
- PMID: 36142746
- PMCID: PMC9504850
- DOI: 10.3390/ijms231810834
Engineering Nanofiber Scaffolds with Biomimetic Cues for Differentiation of Skin-Derived Neural Crest-like Stem Cells to Schwann Cells
Abstract
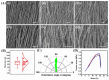
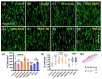
Our laboratory reported the derivation of neural crest stem cell (NCSC)-like cells from the interfollicular epidermis of the neonatal and adult epidermis. These keratinocyte (KC)-derived Neural Crest (NC)-like cells (KC-NC) could differentiate into functional neurons, Schwann cells (SC), melanocytes, and smooth muscle cells in vitro. Most notably, KC-NC migrated along stereotypical pathways and gave rise to multiple NC derivatives upon transplantation into chicken embryos, corroborating their NC phenotype. Here, we present an innovative design concept for developing anisotropically aligned scaffolds with chemically immobilized biological cues to promote differentiation of the KC-NC towards the SC. Specifically, we designed electrospun nanofibers and examined the effect of bioactive cues in guiding KC-NC differentiation into SC. KC-NC attached to nanofibers and adopted a spindle-like morphology, similar to the native extracellular matrix (ECM) microarchitecture of the peripheral nerves. Immobilization of biological cues, especially Neuregulin1 (NRG1) promoted the differentiation of KC-NC into the SC lineage. This study suggests that poly-ε-caprolactone (PCL) nanofibers decorated with topographical and cell-instructive cues may be a potential platform for enhancing KC-NC differentiation toward SC.
Keywords: NRG1; S100b; Schwann cell; keratinocyte derived neural crest; nanofiber; neural crest; poly-ε-caprolactone; proteolipid protein PLP1; topographical cues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Immobilized NRG1 Accelerates Neural Crest like Cell Differentiation Toward Functional Schwann Cells Through Sustained Erk1/2 Activation and YAP/TAZ Nuclear Translocation.Adv Sci (Weinh). 2024 Sep;11(33):e2402607. doi: 10.1002/advs.202402607. Epub 2024 Jul 1. Adv Sci (Weinh). 2024. PMID: 38952126 Free PMC article.
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
-
Neural crest stem cells from human epidermis of aged donors maintain their multipotency in vitro and in vivo.Sci Rep. 2019 Jul 5;9(1):9750. doi: 10.1038/s41598-019-46140-9. Sci Rep. 2019. PMID: 31278326 Free PMC article.
-
Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix.Acta Biomater. 2013 Aug;9(8):7727-36. doi: 10.1016/j.actbio.2013.04.034. Epub 2013 Apr 27. Acta Biomater. 2013. PMID: 23628775
-
Adult tissue-derived neural crest-like stem cells: Sources, regulatory networks, and translational potential.Stem Cells Transl Med. 2020 Mar;9(3):328-341. doi: 10.1002/sctm.19-0173. Epub 2019 Nov 18. Stem Cells Transl Med. 2020. PMID: 31738018 Free PMC article. Review.
Cited by
-
Monocyte Recruitment for Vascular Tissue Regeneration.Adv Healthc Mater. 2022 Nov;11(22):e2200890. doi: 10.1002/adhm.202200890. Epub 2022 Sep 30. Adv Healthc Mater. 2022. PMID: 36112115 Free PMC article.
-
Chitosan Nerve Grafts Incorporated with SKP-SC-EVs Induce Peripheral Nerve Regeneration.Tissue Eng Regen Med. 2023 Apr;20(2):309-322. doi: 10.1007/s13770-022-00517-6. Epub 2023 Mar 6. Tissue Eng Regen Med. 2023. PMID: 36877455 Free PMC article.
-
Immobilized NRG1 Accelerates Neural Crest like Cell Differentiation Toward Functional Schwann Cells Through Sustained Erk1/2 Activation and YAP/TAZ Nuclear Translocation.Adv Sci (Weinh). 2024 Sep;11(33):e2402607. doi: 10.1002/advs.202402607. Epub 2024 Jul 1. Adv Sci (Weinh). 2024. PMID: 38952126 Free PMC article.
-
Injectable shear-thinning hydrogels promote oligodendrocyte progenitor cell survival and remyelination in the central nervous system.Sci Adv. 2024 Jul 12;10(28):eadk9918. doi: 10.1126/sciadv.adk9918. Epub 2024 Jul 12. Sci Adv. 2024. PMID: 38996029 Free PMC article.
-
Cobalt ions-derived nanoenzyme array for endosseous neural network reconstruction and osseointegration.Bioact Mater. 2024 Aug 14;42:1-17. doi: 10.1016/j.bioactmat.2024.08.005. eCollection 2024 Dec. Bioact Mater. 2024. PMID: 39246698 Free PMC article.
References
-
- Mohamed M.A., Fallahi A., El-Sokkary A.M., Salehi S., Akl M.A., Jafari A., Tamayol A., Fenniri H., Khademhosseini A., Andreadis S.T., et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019;98:101147. doi: 10.1016/j.progpolymsci.2019.101147. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

