Cerium Oxide Nanoparticles Regulate Oxidative Stress in HeLa Cells by Increasing the Aquaporin-Mediated Hydrogen Peroxide Permeability
- PMID: 36142747
- PMCID: PMC9506032
- DOI: 10.3390/ijms231810837
Cerium Oxide Nanoparticles Regulate Oxidative Stress in HeLa Cells by Increasing the Aquaporin-Mediated Hydrogen Peroxide Permeability
Abstract
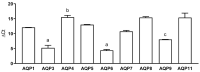
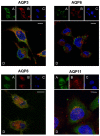
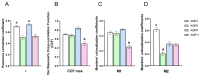
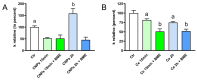
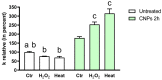
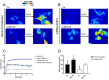
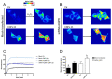
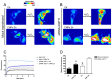
Some aquaporins (AQPs) allow the diffusion of hydrogen peroxide (H2O2), the most abundant ROS, through the cell membranes. Therefore, the possibility of regulating the AQP-mediated permeability to H2O2, and thus ROS scavenging, appears particularly important for controlling the redox state of cells in physiological and pathophysiological conditions. Several compounds have been screened and characterized for this purpose. This study aimed to analyze the effect of cerium oxide nanoparticles (CNPs) presenting antioxidant activity on AQP functioning. HeLa cells express AQP3, 6, 8, and 11, able to facilitate H2O2. AQP3, 6, and 8 are expressed in the plasma membrane and intracellularly, while AQP11 resides only in intracellular structures. CNPs but not cerium ions treatment significantly increased the water and H2O2 permeability by interacting with AQP3, 6, and especially with AQP8. CNPs increased considerably the AQP-mediated water diffusion in cells with oxidative stress. Functional experiments with silenced HeLa cells revealed that CNPs increased the H2O2 diffusion mainly by modulating the AQP8 permeability but also the AQP3 and AQP6, even if to a lesser extent. Current findings suggest that CNPs represent a promising pharmaceutical agent that might potentially be used in numerous pathologies involving oxidative stress as tumors and neurodegenerative diseases.
Keywords: CeO2; HeLa; HyPer7 probe; nanoparticles; peroxiporin; water channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Sigma1 Receptor Modulates Plasma Membrane and Mitochondrial Peroxiporins.Cells. 2025 Jul 15;14(14):1082. doi: 10.3390/cells14141082. Cells. 2025. PMID: 40710335 Free PMC article.
-
Polyacrylic-Coated Solid Nanoparticles Increase the Aquaporin Permeability to Hydrogen Peroxide.Int J Mol Sci. 2023 Dec 27;25(1):372. doi: 10.3390/ijms25010372. Int J Mol Sci. 2023. PMID: 38203543 Free PMC article.
-
Sigma-1 Receptor Agonists Acting on Aquaporin-Mediated H2O2 Permeability: New Tools for Counteracting Oxidative Stress.Int J Mol Sci. 2021 Sep 10;22(18):9790. doi: 10.3390/ijms22189790. Int J Mol Sci. 2021. PMID: 34575952 Free PMC article.
-
Human sperm functioning is related to the aquaporin-mediated water and hydrogen peroxide transport regulation.Biochimie. 2021 Sep;188:45-51. doi: 10.1016/j.biochi.2021.05.011. Epub 2021 Jun 2. Biochimie. 2021. PMID: 34087390 Review.
-
Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide.Biochim Biophys Acta. 2014 May;1840(5):1596-604. doi: 10.1016/j.bbagen.2013.09.017. Epub 2013 Sep 20. Biochim Biophys Acta. 2014. PMID: 24060746 Review.
Cited by
-
Peroxiporins and Oxidative Stress: Promising Targets to Tackle Inflammation and Cancer.Int J Mol Sci. 2024 Aug 1;25(15):8381. doi: 10.3390/ijms25158381. Int J Mol Sci. 2024. PMID: 39125952 Free PMC article. Review.
-
Sigma1 Receptor Modulates Plasma Membrane and Mitochondrial Peroxiporins.Cells. 2025 Jul 15;14(14):1082. doi: 10.3390/cells14141082. Cells. 2025. PMID: 40710335 Free PMC article.
-
Polyacrylic-Coated Solid Nanoparticles Increase the Aquaporin Permeability to Hydrogen Peroxide.Int J Mol Sci. 2023 Dec 27;25(1):372. doi: 10.3390/ijms25010372. Int J Mol Sci. 2023. PMID: 38203543 Free PMC article.
-
Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases.Int J Mol Sci. 2023 Sep 17;24(18):14201. doi: 10.3390/ijms241814201. Int J Mol Sci. 2023. PMID: 37762504 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

