Synthesis, Molecular Docking, In Vitro and In Vivo Studies of Novel Dimorpholinoquinazoline-Based Potential Inhibitors of PI3K/Akt/mTOR Pathway
- PMID: 36142768
- PMCID: PMC9503112
- DOI: 10.3390/ijms231810854
Synthesis, Molecular Docking, In Vitro and In Vivo Studies of Novel Dimorpholinoquinazoline-Based Potential Inhibitors of PI3K/Akt/mTOR Pathway
Abstract
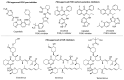
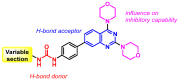
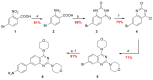
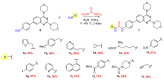
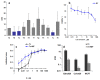
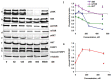
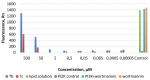
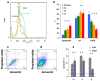
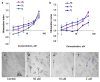
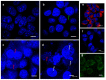
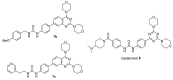
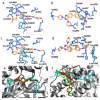
A (series) range of potential dimorpholinoquinazoline-based inhibitors of the PI3K/Akt/mTOR cascade was synthesized. Several compounds exhibited cytotoxicity towards a panel of cancer cell lines in the low and sub-micromolar range. Compound 7c with the highest activity and moderate selectivity towards MCF7 cells which express the mutant type of PI3K was also tested for the ability to inhibit PI3K-(signaling pathway) downstream effectors and associated proteins. Compound 7c inhibited the phosphorylation of Akt, mTOR, and S6K at 125-250 nM. It also triggered PARP1 cleavage, ROS production, and cell death via several mechanisms. Inhibition of PI3Kα was observed at a concentration of 7b 50 µM and of 7c 500 µM and higher, that can indicate minority PI3Kα as a target among other kinases in the titled cascade for 7c. In vivo studies demonstrated an inhibition of tumor growth in the colorectal tumor model. According to the docking studies, the replacement of the triazine core in gedatolisib (8) by a quinazoline fragment, and incorporation of a (hetero)aromatic unit connected with the carbamide group via a flexible spacer, can result in more selective inhibition of the PI3Kα isoform.
Keywords: PI3K/Akt/mTOR inhibitor; S6K; cancer; dimorpholinoquinazoline; kinase inhibition activity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Design, synthesis and pharmacological evaluation of 2-arylurea-1,3,5-triazine derivative (XIN-9): A novel potent dual PI3K/mTOR inhibitor for cancer therapy.Bioorg Chem. 2022 Dec;129:106157. doi: 10.1016/j.bioorg.2022.106157. Epub 2022 Sep 27. Bioorg Chem. 2022. PMID: 36209563
-
Synthesis and structure-activity relationships of dual PI3K/mTOR inhibitors based on a 4-amino-6-methyl-1,3,5-triazine sulfonamide scaffold.Bioorg Med Chem Lett. 2012 Sep 1;22(17):5714-20. doi: 10.1016/j.bmcl.2012.06.078. Epub 2012 Jul 3. Bioorg Med Chem Lett. 2012. PMID: 22832322
-
Synthesis and evaluation of pyrrolotriazine based molecules as PI3 kinase inhibitors.Bioorg Med Chem Lett. 2015 Aug 15;25(16):3142-6. doi: 10.1016/j.bmcl.2015.06.007. Epub 2015 Jun 10. Bioorg Med Chem Lett. 2015. PMID: 26112437
-
The PI3K/AKT/mTOR signaling pathway inhibitors enhance radiosensitivity in cancer cell lines.Mol Biol Rep. 2021 Aug;48(8):1-14. doi: 10.1007/s11033-021-06607-3. Epub 2021 Aug 6. Mol Biol Rep. 2021. PMID: 34357550 Review.
-
PI3K/Akt/mTOR pathway inhibitors in cancer: a perspective on clinical progress.Curr Med Chem. 2010;17(35):4326-41. doi: 10.2174/092986710793361234. Curr Med Chem. 2010. PMID: 20939811 Review.
Cited by
-
Synthesis and evaluation of sulfonamide derivatives of quinoxaline 1,4-dioxides as carbonic anhydrase inhibitors.RSC Adv. 2024 Jul 23;14(32):23257-23272. doi: 10.1039/d4ra04548c. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39045402 Free PMC article.
-
Novel N-(4,5,6,7-tetrahydrobenzisoxazol-4-yl)amides as HSP90 inhibitors: design, synthesis and biological evaluation.RSC Med Chem. 2025 Mar 28. doi: 10.1039/d4md00904e. Online ahead of print. RSC Med Chem. 2025. PMID: 40223823 Free PMC article.
-
iDCNNPred: an interpretable deep learning model for virtual screening and identification of PI3Ka inhibitors against triple-negative breast cancer.Mol Divers. 2025 Aug;29(4):3077-3100. doi: 10.1007/s11030-024-11055-9. Epub 2024 Dec 8. Mol Divers. 2025. PMID: 39648257
-
The antibreast cancer therapeutic potential of quinazoline hybrids-Part I.Future Med Chem. 2025 May;17(9):1055-1069. doi: 10.1080/17568919.2025.2498881. Epub 2025 Apr 30. Future Med Chem. 2025. PMID: 40304260 Review.
-
Synthesis and Biological Evaluation of Chalconesulfonamides: En Route to Proapoptotic Agents with Antiestrogenic Potency.Pharmaceuticals (Basel). 2023 Dec 25;17(1):32. doi: 10.3390/ph17010032. Pharmaceuticals (Basel). 2023. PMID: 38256865 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

