Function-Related Asymmetry of the Interactions between Matrix Loops and Conserved Sequence Motifs in the Mitochondrial ADP/ATP Carrier
- PMID: 36142790
- PMCID: PMC9502086
- DOI: 10.3390/ijms231810877
Function-Related Asymmetry of the Interactions between Matrix Loops and Conserved Sequence Motifs in the Mitochondrial ADP/ATP Carrier
Abstract
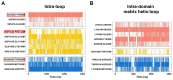
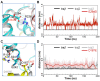
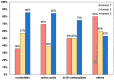
The ADP/ATP carrier (AAC) plays a central role in oxidative metabolism by exchanging ATP and ADP across the inner mitochondrial membrane. Previous experiments have shown the involvement of the matrix loops of AAC in its function, yet potential mechanisms remain largely elusive. One obstacle is the limited information on the structural dynamics of the matrix loops. In the current work, unbiased all-atom molecular dynamics (MD) simulations were carried out on c-state wild-type AAC and mutants. Our results reveal that: (1) two ends of a matrix loop are tethered through interactions between the residue of triplet 38 (Q38, D143 and Q240) located at the C-end of the odd-numbered helix and residues of the [YF]xG motif located before the N-end of the short matrix helix in the same domain; (2) the initial progression direction of a matrix loop is determined by interactions between the negatively charged residue of the [DE]G motif located at the C-end of the short matrix helix and the capping arginine (R30, R139 and R236) in the previous domain; (3) the two chemically similar residues D and E in the highly conserved [DE]G motif are actually quite different; (4) the N-end of the M3 loop is clamped by the [DE]G motif and the capping arginine of domain 2 from the two sides, which strengthens interactions between domain 2 and domain 3; and (5) a highly asymmetric stable core exists within domains 2 and 3 at the m-gate level. Moreover, our results help explain almost all extremely conserved residues within the matrix loops of the ADP/ATP carriers from a structural point of view. Taken together, the current work highlights asymmetry in the three matrix loops and implies a close relationship between asymmetry and ADP/ATP transport.
Keywords: ADP/ATP carrier (AAC); MCF motif; loops; mitochondrial carrier family (MCF); molecular dynamics simulation; transporters.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article.
Figures






Similar articles
-
Molecular dynamics simulations on apo ADP/ATP carrier shed new lights on the featured motif of the mitochondrial carriers.Mitochondrion. 2019 Jul;47:94-102. doi: 10.1016/j.mito.2019.05.006. Epub 2019 May 23. Mitochondrion. 2019. PMID: 31129042
-
The effects of cardiolipin on the structural dynamics of the mitochondrial ADP/ATP carrier in its cytosol-open state.J Lipid Res. 2022 Jun;63(6):100227. doi: 10.1016/j.jlr.2022.100227. Epub 2022 May 12. J Lipid Res. 2022. PMID: 35569528 Free PMC article.
-
Investigating the Broad Matrix-Gate Network in the Mitochondrial ADP/ATP Carrier through Molecular Dynamics Simulations.Molecules. 2022 Feb 5;27(3):1071. doi: 10.3390/molecules27031071. Molecules. 2022. PMID: 35164338 Free PMC article.
-
The ADP and ATP transport in mitochondria and its carrier.Biochim Biophys Acta. 2008 Oct;1778(10):1978-2021. doi: 10.1016/j.bbamem.2008.04.011. Epub 2008 May 2. Biochim Biophys Acta. 2008. PMID: 18510943 Review.
-
Exploring the proton transport mechanism of the mitochondrial ADP/ATP carrier: FA-cycling hypothesis and beyond.Protein Sci. 2025 Mar;34(3):e70047. doi: 10.1002/pro.70047. Protein Sci. 2025. PMID: 39969060 Free PMC article. Review.
Cited by
-
In Silico Analysis of the Structural Dynamics and Substrate Recognition Determinants of the Human Mitochondrial Carnitine/Acylcarnitine SLC25A20 Transporter.Int J Mol Sci. 2023 Feb 15;24(4):3946. doi: 10.3390/ijms24043946. Int J Mol Sci. 2023. PMID: 36835358 Free PMC article.
References
-
- Lamarca V., Sanz-Clemente A., Pérez-Pé R., Martínez-Lorenzo M.J., Halaihel N., Muniesa P., Carrodeguas J.A. Two isoforms of PSAP/MTCH1 share two proapoptotic domains and multiple internal signals for import into the mitochondrial outer membrane. Am. J. Physiol. Cell Physiol. 2007;293:C1347–C1361. doi: 10.1152/ajpcell.00431.2006. - DOI - PubMed
-
- Abrams A.J., Hufnagel R.B., Rebelo A., Zanna C., Patel N., Gonzalez M.A., Campeanu I.J., Griffin L.B., Groenewald S., Strickland A.V., et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat. Genet. 2015;47:926–932. doi: 10.1038/ng.3354. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

