How "Neuronal" Are Human Skin Mast Cells?
- PMID: 36142795
- PMCID: PMC9505265
- DOI: 10.3390/ijms231810871
How "Neuronal" Are Human Skin Mast Cells?
Abstract
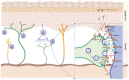
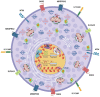
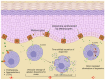
Mast cells are evolutionarily old cells and the principal effectors in allergic responses and inflammation. They are seeded from the yolk sac during embryogenesis or are derived from hematopoietic progenitors and are therefore related to other leukocyte subsets, even though they form a separate clade in the hematopoietic system. Herein, we systematically bundle information from several recent high-throughput endeavors, especially those comparing MCs with other cell types, and combine such information with knowledge on the genes' functions to reveal groups of neuronal markers specifically expressed by MCs. We focus on recent advances made regarding human tissue MCs, but also refer to studies in mice. In broad terms, genes hyper-expressed in MCs, but largely inactive in other myelocytes, can be classified into subcategories such as traffic/lysosomes (MLPH and RAB27B), the dopamine system (MAOB, DRD2, SLC6A3, and SLC18A2), Ca2+-related entities (CALB2), adhesion molecules (L1CAM and NTM) and, as an overall principle, the transcription factors and modulators of transcriptional activity (LMO4, PBX1, MEIS2, and EHMT2). Their function in MCs is generally unknown but may tentatively be deduced by comparison with other systems. MCs share functions with the nervous system, as they express typical neurotransmitters (histamine and serotonin) and a degranulation machinery that shares features with the neuronal apparatus at the synapse. Therefore, selective overlaps are plausible, and they further highlight the uniqueness of MCs within the myeloid system, as well as when compared with basophils. Apart from investigating their functional implications in MCs, a key question is whether their expression in the lineage is due to the specific reactivation of genes normally silenced in leukocytes or whether the genes are not switched off during mastocytic development from early progenitors.
Keywords: adhesion molecules; calbindin; degranulation; dopamine; mast cells; monoamine oxidase B; neurons; skin; solute carriers; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
IL-4 and human skin mast cells revisited: reinforcement of a pro-allergic phenotype upon prolonged exposure.Arch Dermatol Res. 2016 Nov;308(9):665-670. doi: 10.1007/s00403-016-1688-x. Epub 2016 Sep 20. Arch Dermatol Res. 2016. PMID: 27650274
-
Etv2 is expressed in the yolk sac hematopoietic and endothelial progenitors and regulates Lmo2 gene expression.Stem Cells. 2012 Aug;30(8):1611-23. doi: 10.1002/stem.1131. Stem Cells. 2012. PMID: 22628281 Free PMC article.
-
How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells? A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells.Cells. 2020 Sep 17;9(9):2118. doi: 10.3390/cells9092118. Cells. 2020. PMID: 32957735 Free PMC article.
-
Mast Cell Serotonin Immunoregulatory Effects Impacting on Neuronal Function: Implications for Neurodegenerative and Psychiatric Disorders.Neurotox Res. 2015 Aug;28(2):147-53. doi: 10.1007/s12640-015-9533-0. Epub 2015 Jun 3. Neurotox Res. 2015. PMID: 26038194 Review.
-
Histamine Release from Mast Cells and Basophils.Handb Exp Pharmacol. 2017;241:121-139. doi: 10.1007/164_2017_18. Handb Exp Pharmacol. 2017. PMID: 28332048 Review.
Cited by
-
Cultures of Human Skin Mast Cells, an Attractive In Vitro Model for Studies of Human Mast Cell Biology.Cells. 2024 Jan 2;13(1):98. doi: 10.3390/cells13010098. Cells. 2024. PMID: 38201301 Free PMC article.
-
Characterization of Freshly Isolated Human Peripheral Blood B Cells, Monocytes, CD4+ and CD8+ T Cells, and Skin Mast Cells by Quantitative Transcriptomics.Int J Mol Sci. 2024 Dec 4;25(23):13050. doi: 10.3390/ijms252313050. Int J Mol Sci. 2024. PMID: 39684762 Free PMC article.
-
Editorial of Special Issue "Molecular Mechanisms of Allergy and Asthma 2.0".Int J Mol Sci. 2023 Jul 11;24(14):11310. doi: 10.3390/ijms241411310. Int J Mol Sci. 2023. PMID: 37511070 Free PMC article.
-
Clorfl86/RHEX Is a Negative Regulator of SCF/KIT Signaling in Human Skin Mast Cells.Cells. 2023 May 3;12(9):1306. doi: 10.3390/cells12091306. Cells. 2023. PMID: 37174705 Free PMC article.
-
CREB Is Activated by the SCF/KIT Axis in a Partially ERK-Dependent Manner and Orchestrates Survival and the Induction of Immediate Early Genes in Human Skin Mast Cells.Int J Mol Sci. 2023 Feb 18;24(4):4135. doi: 10.3390/ijms24044135. Int J Mol Sci. 2023. PMID: 36835547 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

