Sequential Targeting of PLK1 and PARP1 Reverses the Resistance to PARP Inhibitors and Enhances Platin-Based Chemotherapy in BRCA-Deficient High-Grade Serous Ovarian Cancer with KRAS Amplification
- PMID: 36142803
- PMCID: PMC9502276
- DOI: 10.3390/ijms231810892
Sequential Targeting of PLK1 and PARP1 Reverses the Resistance to PARP Inhibitors and Enhances Platin-Based Chemotherapy in BRCA-Deficient High-Grade Serous Ovarian Cancer with KRAS Amplification
Abstract
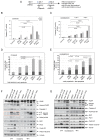
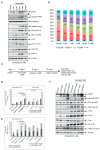
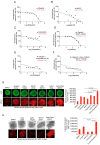
Ovarian cancer (OC) accounts for approximately 4% of cancer deaths in women worldwide and is the deadliest gynecologic malignancy. High-grade serous ovarian cancer (HGSOC) is the most predominant ovarian cancer, in which BRCA1/2 gene mutation ranges from 3 to 27%. PARP inhibitors (PARPi) have shown promising results as a synthetically lethal therapeutic approach for BRCA mutant and recurrent OC in clinical use. However, emerging data indicate that BRCA-deficient cancers may be resistant to PARPi, and the mechanisms of this resistance remain elusive. We found that amplification of KRAS likely underlies PARPi resistance in BRCA2-deficient HGSOC. Our data suggest that PLK1 inhibition restores sensitivity to PARPi in HGSOC with KRAS amplification. The sequential combination of PLK1 inhibitor (PLK1i) and PARPi drastically reduces HGSOC cell survival and increases apoptosis. Furthermore, we were able to show that a sequential combination of PLK1i and PARPi enhanced the cellular apoptotic response to carboplatin-based chemotherapy in KRAS-amplified resistant HGSOC cells and 3D spheroids derived from recurrent ovarian cancer patients. Our results shed new light on the critical role of PLK1 in reversing PARPi resistance in KRAS-amplified HGSOC, and offer a new therapeutic strategy for this class of ovarian cancer patients where only limited options currently exist.
Keywords: BRCA2 deficiency; DNA damage; KRAS amplification; PARP inhibitor resistance; PLK1-based combinatorial therapy; high-grade serous ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Histone methyltransferases EHMT1 and EHMT2 (GLP/G9A) maintain PARP inhibitor resistance in high-grade serous ovarian carcinoma.Clin Epigenetics. 2019 Nov 27;11(1):165. doi: 10.1186/s13148-019-0758-2. Clin Epigenetics. 2019. PMID: 31775874 Free PMC article.
-
The disruption of the CCDC6 - PP4 axis induces a BRCAness like phenotype and sensitivity to PARP inhibitors in high-grade serous ovarian carcinoma.J Exp Clin Cancer Res. 2022 Aug 13;41(1):245. doi: 10.1186/s13046-022-02459-2. J Exp Clin Cancer Res. 2022. PMID: 35964058 Free PMC article.
-
Activation of Wnt signaling promotes olaparib resistant ovarian cancer.Mol Carcinog. 2019 Oct;58(10):1770-1782. doi: 10.1002/mc.23064. Epub 2019 Jun 10. Mol Carcinog. 2019. PMID: 31219654 Free PMC article.
-
PARP inhibitors in platinum-sensitive high-grade serous ovarian cancer.Cancer Chemother Pharmacol. 2018 Apr;81(4):647-658. doi: 10.1007/s00280-018-3532-9. Epub 2018 Feb 20. Cancer Chemother Pharmacol. 2018. PMID: 29464354 Free PMC article. Review.
-
Overcoming PARPi resistance: Preclinical and clinical evidence in ovarian cancer.Drug Resist Updat. 2021 Mar;55:100744. doi: 10.1016/j.drup.2021.100744. Epub 2021 Jan 16. Drug Resist Updat. 2021. PMID: 33551306 Review.
Cited by
-
Onvansertib treatment overcomes olaparib resistance in high-grade ovarian carcinomas.Cell Death Dis. 2024 Jul 22;15(7):521. doi: 10.1038/s41419-024-06894-1. Cell Death Dis. 2024. PMID: 39039067 Free PMC article.
-
MLN0905 effectively kills gemcitabine-resistant pancreatic cancer cells by targeting PLK1.Eur J Med Res. 2025 Jul 3;30(1):567. doi: 10.1186/s40001-025-02825-8. Eur J Med Res. 2025. PMID: 40611188 Free PMC article.
-
PARP Inhibitors and Proteins Interacting with SLX4.Cancers (Basel). 2023 Feb 3;15(3):997. doi: 10.3390/cancers15030997. Cancers (Basel). 2023. PMID: 36765954 Free PMC article. Review.
-
Exploiting Cancer Dormancy Signaling Mechanisms in Epithelial Ovarian Cancer Through Spheroid and Organoid Analysis.Cells. 2025 Jan 17;14(2):133. doi: 10.3390/cells14020133. Cells. 2025. PMID: 39851561 Free PMC article.
-
PLK1 and PARP positively correlate in Middle Eastern breast cancer and their combined inhibition overcomes PARP inhibitor resistance in triple negative breast cancer.Front Oncol. 2024 Jan 3;13:1286585. doi: 10.3389/fonc.2023.1286585. eCollection 2023. Front Oncol. 2024. PMID: 38234395 Free PMC article.
References
-
- Hoskins W.J., McGuire W.P., Brady M.F., Homesley H.D., Creasman W.T., Berman M., Ball H., Berek J.S. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am. J. Obstet. Gynecol. 1994;170:974–979; discussion 979–980. doi: 10.1016/S0002-9378(94)70090-7. - DOI - PubMed
-
- Ozols R.F., Bundy B.N., Greer B.E., Fowler J.M., Clarke-Pearson D., Burger R.A., Mannel R.S., DeGeest K., Hartenbach E.M., Baergen R., et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

