Plasmids as Key Players in Acinetobacter Adaptation
- PMID: 36142804
- PMCID: PMC9501444
- DOI: 10.3390/ijms231810893
Plasmids as Key Players in Acinetobacter Adaptation
Abstract
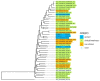
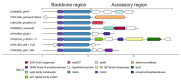
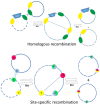
This review briefly summarizes the data on the mechanisms of development of the adaptability of Acinetobacters to various living conditions in the environment and in the clinic. A comparative analysis of the genomes of free-living and clinical strains of A. lwoffii, as well as the genomes of A. lwoffii and A. baumannii, has been carried out. It has been shown that plasmids, both large and small, play a key role in the formation of the adaptability of Acinetobacter to their living conditions. In particular, it has been demonstrated that the plasmids of various strains of Acinetobacter differ from each other in their structure and gene composition depending on the lifestyle of their host bacteria. Plasmids of modern strains are enriched with antibiotic-resistant genes, while the content of genes involved in resistance to heavy metals and arsenic is comparable to plasmids from modern and ancient strains. It is concluded that Acinetobacter plasmids may ensure the survival of host bacteria under conditions of various types of environmental and clinical stresses. A brief overview of the main mechanisms of horizontal gene transfer on plasmids inherent in Acinetobacter strains is also given.
Keywords: accessory region; antibiotic resistance; heavy metals resistance; plasmid backbone; recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lee C.R., Lee J.H., Park M., Park K.S., Bae I.K., Kim Y.B., Cha C.J., Jeong B.C., Jee S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

