The Controversial Role of LPS in Platelet Activation In Vitro
- PMID: 36142813
- PMCID: PMC9505944
- DOI: 10.3390/ijms231810900
The Controversial Role of LPS in Platelet Activation In Vitro
Abstract
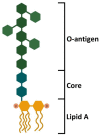
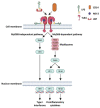
Circulating platelets are responsible for hemostasis and thrombosis but are also primary sensors of pathogens and are involved in innate immunity, inflammation, and sepsis. Sepsis is commonly caused by an exaggerated immune response to bacterial, viral, and fungal infections, and leads to severe thrombotic complications. Among others, the endotoxin lipopolysaccharide (LPS) found in the outer membrane of Gram-negative bacteria is the most common trigger of sepsis. Since the discovery of the expression of the LPS receptor TLR4 in platelets, several studies have investigated the ability of LPS to induce platelet activation and to contribute to a prothrombotic phenotype, per se or in combination with plasma proteins and platelet agonists. This issue, however, is still controversial, as different sources, purity, and concentrations of LPS, different platelet-purification protocols, and different methods of analysis have been used in the past two decades, giving contradictory results. This review summarizes and critically analyzes past and recent publications about LPS-induced platelet activation in vitro. A methodological section illustrates the principal platelet preparation protocols and significant differences. The ability of various sources of LPS to elicit platelet activation in terms of aggregation, granule secretion, cytokine release, ROS production, and interaction with leukocytes and NET formation is discussed.
Keywords: E. coli; LPS; TLR4; platelets; sepsis; thromboinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

