Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension
- PMID: 36142826
- PMCID: PMC9501428
- DOI: 10.3390/ijms231810912
Skeletal Muscle Dysfunction in Experimental Pulmonary Hypertension
Abstract
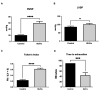
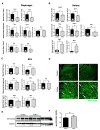
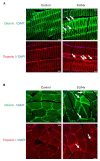
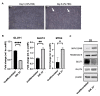
Pulmonary arterial hypertension (PAH) is a serious, progressive, and often fatal disease that is in urgent need of improved therapies that treat it. One of the remaining therapeutic challenges is the increasingly recognized skeletal muscle dysfunction that interferes with exercise tolerance. Here we report that in the adult rat Sugen/hypoxia (SU/Hx) model of severe pulmonary hypertension (PH), there is highly significant, almost 50%, decrease in exercise endurance, and this is associated with a 25% increase in the abundance of type II muscle fiber markers, thick sarcomeric aggregates and an increase in the levels of FoxO1 in the soleus (a predominantly type I fiber muscle), with additional alterations in the transcriptomic profiles of the diaphragm (a mixed fiber muscle) and the extensor digitorum longus (a predominantly Type II fiber muscle). In addition, soleus atrophy may contribute to impaired exercise endurance. Studies in L6 rat myoblasts have showed that myotube differentiation is associated with increased FoxO1 levels and type II fiber markers, while the inhibition of FoxO1 leads to increased type I fiber markers. We conclude that the formation of aggregates and a FoxO1-mediated shift in the skeletal muscle fiber-type specification may underlie skeletal muscle dysfunction in an experimental study of PH.
Keywords: FoxO1; PAH; skeletal muscle; type II fibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Effect of endurance training on oestrogen receptor alpha expression in different rat skeletal muscle type.Acta Physiol Scand. 2002 Jul;175(3):211-7. doi: 10.1046/j.1365-201X.2002.00992.x. Acta Physiol Scand. 2002. PMID: 12100360
-
Strenuous exercise-induced alterations of muscle fiber cross-sectional area and fiber-type distribution in steroid myopathy rats.Am J Phys Med Rehabil. 2008 Feb;87(2):126-33. doi: 10.1097/PHM.0b013e31815869d0. Am J Phys Med Rehabil. 2008. PMID: 17993993
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
-
Altered skeletal muscle function and beneficial effects of exercise training in a rat model of induced pulmonary emphysema.Acta Physiol (Oxf). 2024 Jul;240(7):e14165. doi: 10.1111/apha.14165. Epub 2024 May 15. Acta Physiol (Oxf). 2024. PMID: 38747536
-
FoxO1: a novel insight into its molecular mechanisms in the regulation of skeletal muscle differentiation and fiber type specification.Oncotarget. 2017 Feb 7;8(6):10662-10674. doi: 10.18632/oncotarget.12891. Oncotarget. 2017. PMID: 27793012 Free PMC article. Review.
Cited by
-
Skeletal and cardiac muscle have different protein turnover responses in a model of right heart failure.Geroscience. 2023 Aug;45(4):2545-2557. doi: 10.1007/s11357-023-00777-7. Epub 2023 Apr 29. Geroscience. 2023. PMID: 37118350 Free PMC article.
-
Honokiol and Nicotinamide Adenine Dinucleotide Improve Exercise Endurance in Pulmonary Hypertensive Rats Through Increasing SIRT3 Function in Skeletal Muscle.Int J Mol Sci. 2024 Oct 29;25(21):11600. doi: 10.3390/ijms252111600. Int J Mol Sci. 2024. PMID: 39519152 Free PMC article.
-
Pulmonary hypertension alters blood flow distribution and impairs the hyperemic response in the rat diaphragm.Front Physiol. 2023 Dec 22;14:1281715. doi: 10.3389/fphys.2023.1281715. eCollection 2023. Front Physiol. 2023. PMID: 38187132 Free PMC article.
-
Impact of Aerobic Training on Transcriptomic Changes in Skeletal Muscle of Rats with Cardiac Cachexia.Int J Mol Sci. 2025 Jul 7;26(13):6525. doi: 10.3390/ijms26136525. Int J Mol Sci. 2025. PMID: 40650303 Free PMC article.
-
Beyond Tumors: The Pivotal Role of TRIM Proteins in Chronic Non-Tumor Lung Diseases.J Inflamm Res. 2025 Feb 7;18:1899-1910. doi: 10.2147/JIR.S499029. eCollection 2025. J Inflamm Res. 2025. PMID: 39935527 Free PMC article. Review.
References
-
- Enache I., Charles A.-L., Bouitbir J., Favret F., Zoll J., Metzger D., Oswald-Mammosser M., Geny B., Charloux A. Skeletal muscle mitochondrial dysfunction precedes right ventricular impairment in experimental pulmonary hypertension. Mol. Cell. Biochem. 2013;373:161–170. doi: 10.1007/s11010-012-1485-6. - DOI - PubMed
-
- Gonçalves D., Padrão A.I., Ferreira R., Justino J., Neuparth M.J., Vitorino R., Fonseca H., Silva A.F., Duarte J.A., Leite-Moreira A., et al. Signaling pathways underlying skeletal muscle wasting in experimental pulmonary arterial hypertension. Biochim. Biophys. Acta. 2015;1852:2722–2731. doi: 10.1016/j.bbadis.2015.10.002. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

