Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review
- PMID: 36142866
- PMCID: PMC9502843
- DOI: 10.3390/ijms231810948
Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: A Systematic Review
Abstract
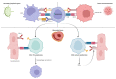
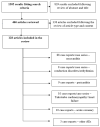
Immune checkpoint inhibitors (ICIs) are an important advancement in the field of cancer treatment, significantly improving the survival of patients with a series of advanced malignancies, like melanoma, non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), and Hodgkin lymphoma. ICIs act upon T lymphocytes and antigen-presenting cells, targeting programmed cell death protein 1 (PD1), programmed cell death protein ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4), breaking the immune tolerance of the T cells against malignant cells and enhancing the body's own immune response. A variety of cardiac-adverse effects are associated with ICI-based treatment, including pericarditis, arrhythmias, cardiomyopathy, and acute coronary syndrome, with myocarditis being the most studied due to its often-unexpected onset and severity. Overall, Myocarditis is rare but presents an immune-related adverse event (irAE) that has a high fatality rate. Considering the rising number of oncological patients treated with ICIs and the severity of their potential adverse effects, a good understanding and continuous investigation of cardiac irAEs is of the utmost importance. This systematic review aimed to revise recent publications (between 2016-2022) on ICI-induced cardiac toxicities and highlight the therapeutical approach and evolution in the selected cases.
Keywords: arrythmia; cardiomyopathy; immune checkpoint inhibitors; myocarditis; pericarditis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Puzanov I., Diab A., Abdallah K., Bingham C.O., Brogdon C., Dadu R., Hamad L., Kim S., Lacouture M.E., LeBoeuf N.R., et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials