Human Milk Oligosaccharide 2'-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells
- PMID: 36142892
- PMCID: PMC9506168
- DOI: 10.3390/ijms231810958
Human Milk Oligosaccharide 2'-Fucosyllactose Modulates Local Viral Immune Defense by Supporting the Regulatory Functions of Intestinal Epithelial and Immune Cells
Abstract
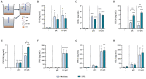
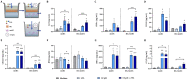
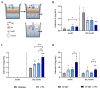
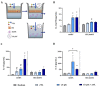
Human milk contains bioactive components that provide protection against viral infections in early life. In particular, intestinal epithelial cells (IEC) have key regulatory roles in the prevention of enteric viral infections. Here we established an in vitro model to study the modulation of host responses against enteric viruses mimicked by poly I:C (pIC). The effects of 2'-fucosyllactose (2'FL), abundantly present in human milk, were studied on IEC and/or innate immune cells, and the subsequent functional response of the adaptive immune cells. IEC were pre-incubated with 2'FL and stimulated with naked or Lyovec™-complexed pIC (LV-pIC). Additionally, monocyte-derived dendritic cells (moDC) alone or in co-culture with IEC were stimulated with LV-pIC. Then, conditioned-moDC were co-cultured with naïve CD4+ T helper (Th)-cells. IEC stimulation with naked or LV-pIC promoted pro-inflammatory IL-8, CCL20, GROα and CXCL10 cytokine secretion. However, only exposure to LV-pIC additionally induced IFNβ, IFNλ1 and CCL5 secretion. Pre-incubation with 2'FL further increased pIC induced CCL20 secretion and LV-pIC induced CXCL10 secretion. LV-pIC-exposed IEC/moDC and moDC cultures showed increased secretion of IL-8, GROα, IFNλ1 and CXCL10, and in the presence of 2'FL galectin-4 and -9 were increased. The LV-pIC-exposed moDC showed a more pronounced secretion of CCL20, CXCL10 and CCL5. The moDC from IEC/moDC cultures did not drive T-cell development in moDC/T-cell cultures, while moDC directly exposed to LV-pIC secreted Th1 driving IL-12p70 and promoted IFNγ secretion by Th-cells. Hereby, a novel intestinal model was established to study mucosal host-defense upon a viral trigger. IEC may support intestinal homeostasis, regulating local viral defense which may be modulated by 2'FL. These results provide insights regarding the protective capacity of human milk components in early life.
Keywords: 2′-fucosyllactose; T-cell; host-defense; moDC; poly I:C; viral infection.
Conflict of interest statement
None of the authors have a competing financial interest in relation to the presented work; J.G. is head of the division of Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of Science at Utrecht University, and partly employed by Danone Nutricia Research B.V., B.v.L. and N.K. are employed by Danone Nutricia Research B.V., B.v.L. is affiliated at and leading a strategic alliance between University Medical Centre Utrecht/Wilhelmina Children’s Hospital and Danone Nutricia Research B.V.
Figures
Similar articles
-
Epithelial-derived galectin-9 containing exosomes contribute to the immunomodulatory effects promoted by 2'-fucosyllactose and short-chain galacto- and long-chain fructo-oligosaccharides.Front Immunol. 2022 Dec 22;13:1026031. doi: 10.3389/fimmu.2022.1026031. eCollection 2022. Front Immunol. 2022. PMID: 36685520 Free PMC article.
-
Ovalbumin-Induced Epithelial Activation Directs Monocyte-Derived Dendritic Cells to Instruct Type 2 Inflammation in T Cells Which Is Differentially Modulated by 2'-Fucosyllactose and 3-Fucosyllactose.J Innate Immun. 2023;15(1):222-239. doi: 10.1159/000526528. Epub 2022 Oct 10. J Innate Immun. 2023. PMID: 36215948 Free PMC article.
-
Exposure of Intestinal Epithelial Cells to 2'-Fucosyllactose and CpG Enhances Galectin Release and Instructs Dendritic Cells to Drive Th1 and Regulatory-Type Immune Development.Biomolecules. 2020 May 19;10(5):784. doi: 10.3390/biom10050784. Biomolecules. 2020. PMID: 32438601 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity.Ann Nutr Metab. 2016;69 Suppl 2(Suppl 2):42-51. doi: 10.1159/000452818. Epub 2017 Jan 20. Ann Nutr Metab. 2016. PMID: 28103609 Free PMC article. Review.
Cited by
-
Human milk oligosaccharides and the association with microbiota in colostrum: a pilot study.Arch Microbiol. 2024 Jan 8;206(2):58. doi: 10.1007/s00203-023-03787-3. Arch Microbiol. 2024. PMID: 38191870 Free PMC article.
-
Interactions of human milk oligosaccharides with the immune system.Front Immunol. 2025 Jan 14;15:1523829. doi: 10.3389/fimmu.2024.1523829. eCollection 2024. Front Immunol. 2025. PMID: 39877362 Free PMC article. Review.
-
Exploring galectin interactions with human milk oligosaccharides and blood group antigens identifies BGA6 as a functional galectin-4 ligand.J Biol Chem. 2024 Aug;300(8):107573. doi: 10.1016/j.jbc.2024.107573. Epub 2024 Jul 14. J Biol Chem. 2024. PMID: 39009340 Free PMC article.
-
Human milk oligosaccharides differentially support gut barrier integrity and enhance Th1 and Th17 cell effector responses in vitro.Front Immunol. 2024 Mar 6;15:1359499. doi: 10.3389/fimmu.2024.1359499. eCollection 2024. Front Immunol. 2024. PMID: 38510254 Free PMC article.
-
Maternal knowledge, attitudes, and practices regarding breastfeeding and mastitis.BMC Pregnancy Childbirth. 2025 Jul 10;25(1):748. doi: 10.1186/s12884-025-07867-8. BMC Pregnancy Childbirth. 2025. PMID: 40640753 Free PMC article.
References
-
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. - DOI - PMC - PubMed
-
- Troeger C., Blacker B.F., Khalil I.A., Rao P.C., Cao S., Zimsen S.R., Albertson S.B., Stanaway J.D., Deshpande A., Abebe Z., et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

