Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study
- PMID: 36143059
- PMCID: PMC9506337
- DOI: 10.3390/jcm11185412
Individual Trajectories of Bone Mineral Density Reveal Persistent Bone Loss in Bone Sarcoma Patients: A Retrospective Study
Abstract
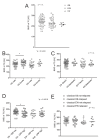
Multiagent chemotherapy offers an undoubted therapeutic benefit to cancer patients, but is also associated with chronic complications in survivors. Osteoporosis affects the quality of life of oncologic patients, especially at the paediatric age. However, very few studies have described the extent of loss of bone mineral density (BMD) in bone sarcoma patients. We analysed a retrospective series of children and adolescents with primary malignant bone tumours (52 osteosarcoma and 31 Ewing sarcoma) and retrieved their BMD at diagnosis and follow-up as Hounsfield units (HU). We studied their individual BMD trajectories before and after chemotherapy up to 5 years, using routine chest CT scan and attenuation thresholds on T12 vertebrae ROI. At one year, bone sarcoma patients showed significant bone loss compared to diagnosis: 17.6% and 17.1% less for OS and EW, respectively. Furthermore, a bone loss of more than 49.2 HU at one-year follow-up was predictive of the persistence of a reduced bone mass over the following 4 years, especially in patients with EW. At 4 years, only 26% and 12.5% of OS and EW, respectively, had recovered or improved their BMD with respect to the onset, suggesting a risk of developing morbidities related to a low BMD in those subjects.
Keywords: BMD; bone sarcoma; cancer survivors; chemotherapy; computed tomography; osteopenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

