B-Cell Targeted Therapies in Patients with Multiple Sclerosis and Incidence of Headache: A Systematic Review and Meta-Analysis
- PMID: 36143259
- PMCID: PMC9504525
- DOI: 10.3390/jpm12091474
B-Cell Targeted Therapies in Patients with Multiple Sclerosis and Incidence of Headache: A Systematic Review and Meta-Analysis
Abstract
Background: Multiple Sclerosis treatment with B-cell targeted therapies may be associated with an increased incidence of headache. We aimed to find and compare the association of B-cell targeted therapies with the incidence of headache in patients with Multiple Sclerosis.
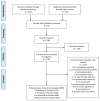
Methods: In a systematic based approach, the following databases were searched from inception until the 6th of June 2020: Pubmed/MEDLINE, ClinicalTrials.gov, EU Clinical Trials Register. Only randomized clinical trials (RCTs) enrolling patients with Multiple Sclerosis comparing B-cell targeted therapies (Rituximab, Ocrelizumab, Ofatumumab, Ublituximab or Cladribine) with placebo were selected for the systematic review and further meta-analysis. PRISMA guidelines were followed at all stages of the systematic review. The primary outcome was an all-cause headache of B-cell targeting therapy in patients with Multiple Sclerosis.
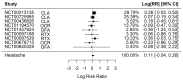
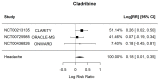
Results: Nine RCTs were included. Compared with placebo, treatment with B-cell targeting therapies revealed a trend in headache risk, but it was not statistically significant (Relative Risk 1.12 [95% Confidence Interval 0.96-1.30]; p = 0.15; I2 = 9.32%). Surprisingly, in a sub-group analysis, Cladribine was statistically significant for an increase in headache risk (RR 1.20 [95% CI 1.006-1.42]; p = 0.042; I2 = 0%; 3 studies with 2107 participants).
Conclusions: Even though a trend is shown, B-cell targeted therapies do not correlate with an increased incidence of headache as an adverse effect. Sub-analyses revealed a significant association between Cladribine alone and an increased incidence of headache. Whereas a purinergic signaling cascade is proposed as a mechanism of action, further research is needed to unravel the underlying pathogenetic mechanism of headache induction and establish headache prevention strategies.
Keywords: B-cell targeted therapies; cladribine; headache incidence; ocrelizumab; ofatumumab; purinergic signaling; rituximab; ublituximab.
Conflict of interest statement
T.M. received travel grants from Merck and Sanofi-Genzyme. M.B. received travel grants from Merck, Teva-Specifar, Genesis Pharma and Pfizer. N.P. declares no conflict of interest. K.P. declares no conflict of interest. A.D. declares no conflict of interest. A.L. declares no conflict of interest. G.V. declares no conflict of interest. A.B.P. declares no conflict of interest. I.P. declares no conflict of interest. D.D.M. received consulting, research, speaking fees and/or travel grants from Allergan, Amgen, Bayer, Biogen, Cefaly, ElectroCore, Eli-Lily, Genesis Pharma, Merck-Serono, Merz, Mylan, Novartis, Roche, Sanofi-Genzyme and Teva-Specifar.
Figures
References
-
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Steiner T.J., Jensen R., Katsarava Z., Linde M., MacGregor E.A., Osipova V., Paemeleire K., Olesen J., Peters M., Martelletti P. Aids to Management of Headache Disorders in Primary Care (2nd Edition): On Behalf of the European Headache Federation and Lifting the Burden: The Global Campaign against Headache. J. Headache Pain. 2019;20:57. doi: 10.1186/s10194-018-0899-2. - DOI - PMC - PubMed
-
- Murray L.C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., et al. Disability-Adjusted Life Years (Dalys) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

