Plasma Angiotensin Converting Enzyme 2 (ACE2) Activity in Healthy Controls and Patients with Cardiovascular Risk Factors and/or Disease
- PMID: 36143280
- PMCID: PMC9501250
- DOI: 10.3390/jpm12091495
Plasma Angiotensin Converting Enzyme 2 (ACE2) Activity in Healthy Controls and Patients with Cardiovascular Risk Factors and/or Disease
Abstract
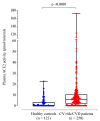
Angiotensin converting enzyme 2 (ACE2) is an endogenous negative regulator of the renin-angiotensin system, a key factor in the development of cardiovascular disease (CVD). ACE2 is also used by SARS-CoV-2 for host cell entry. Given that COVID-19 is associated with hypercoagulability, it is timely to explore the potential relationship between plasma ACE2 activity and the coagulation profile. In this cross-sectional study, ACE2 activity and global coagulation assays (GCA) including thromboelastography, thrombin, and fibrin generation were measured in adult healthy controls (n = 123; mean age 41 ± 17 years; 35% male) and in patients with cardiovascular risk factors and/or disease (n = 258; mean age 65 ± 14 years; 55% male). ACE2 activity was significantly lower in controls compared to patients with cardiovascular risk factors and/or disease (median 0.10 (0.02, 3.33) vs. 5.99 (1.95, 10.37) pmol/mL/min, p < 0.001). Of the healthy controls, 48% had undetectable ACE2 activity. Controls with detectable ACE2 had lower maximum amplitude (p < 0.001). In patients with cardiovascular risk factors and/or disease, those in the 3rd tertile were older and male (p = 0.002), with a higher Framingham grade and increased number of cardiovascular risk factors (p < 0.001). In conclusion, plasma ACE2 activity is undetectable to very low in young healthy controls with minimal clinically relevant associations to GCA. Patients with cardiovascular risk factors and/or disease have increased plasma ACE2 activity, suggesting that it may be an important biomarker of endothelial dysfunction and atherosclerosis.
Keywords: angiotensin converting enzyme 2; cardiovascular disease; coagulation; renin angiotensin system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Emerging markers in cardiovascular disease: where does angiotensin-converting enzyme 2 fit in?Clin Exp Pharmacol Physiol. 2013 Aug;40(8):551-9. doi: 10.1111/1440-1681.12069. Clin Exp Pharmacol Physiol. 2013. PMID: 23432153 Review.
-
Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease.Nephrol Dial Transplant. 2013 Sep;28(9):2287-94. doi: 10.1093/ndt/gft038. Epub 2013 Mar 27. Nephrol Dial Transplant. 2013. PMID: 23535224 Free PMC article.
-
Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors.Eur Heart J. 2020 May 14;41(19):1810-1817. doi: 10.1093/eurheartj/ehaa373. Eur Heart J. 2020. PMID: 32388565 Free PMC article.
-
Angiotensin-converting enzyme 2: a double-edged sword in COVID-19 patients with an increased risk of heart failure.Heart Fail Rev. 2021 Mar;26(2):371-380. doi: 10.1007/s10741-020-10016-2. Heart Fail Rev. 2021. PMID: 32844337 Free PMC article. Review.
-
Strong Association of Angiotensin Converting Enzyme-2 Gene Insertion/Deletion Polymorphism with Susceptibility to SARS-CoV-2, Hypertension, Coronary Artery Disease and COVID-19 Disease Mortality.J Pers Med. 2021 Oct 27;11(11):1098. doi: 10.3390/jpm11111098. J Pers Med. 2021. PMID: 34834450 Free PMC article.
Cited by
-
Persistent Vascular Complications in Long COVID: The Role of ACE2 Deactivation, Microclots, and Uniform Fibrosis.Infect Dis Rep. 2024 Jun 27;16(4):561-571. doi: 10.3390/idr16040042. Infect Dis Rep. 2024. PMID: 39051242 Free PMC article.
References
-
- Patel S.K., Juno J.A., Lee W., Wragg K.M., Hogarth P.M., Kent S.J., Burrell L.M. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: Implications for COVID-19 pathogenesis and consequences. Eur. Respir. J. 2021;57:2003730. doi: 10.1183/13993003.03730-2020. - DOI - PMC - PubMed
-
- Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous