Temperature Increase and Damage Extent at Retinal Pigment Epithelium Compared between Continuous Wave and Micropulse Laser Application
- PMID: 36143352
- PMCID: PMC9504342
- DOI: 10.3390/life12091313
Temperature Increase and Damage Extent at Retinal Pigment Epithelium Compared between Continuous Wave and Micropulse Laser Application
Abstract
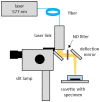
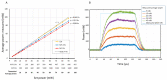
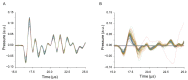
Continuous wave (CW) and microsecond pulse (MP) laser irradiations were compared regarding cell damage and laser-induced temperature rise at retinal pigment epithelium (RPE). The RPE of porcine RPE-choroid-sclera explants was irradiated with a 577 nm laser in CW or MP mode (5% or 15% duty cycle (DC)) for 20 ms or 200 ms at an average laser power of 20−90 mW. Cell viability was investigated with calcein-AM staining. Optoacoustic (OA) technique was employed for temperature measurement during irradiation. For 200 ms irradiation, the dead cell area (DCA) increased linearly (≈1600 µm2/mW) up to the average power of 40 mW for all modes without significant difference. From 50 mW, the increase of DCA of MP-5% significantly dropped to 610 µm2/mW (p < 0.05), likely due to the detected microbubble formation. OA temperature measurement showed a monotonic temperature increase in CW mode and a stepwise increase in MP mode, but no significant difference in the average temperature increase at the same average power, consistent with the temperature modeling. In conclusion, there is no difference in the average temperature rise between CW and MP modes at the same average power regardless of DC. At lower DC, however, more caution is required regarding mechanical damage due to microbubble formation.
Keywords: continuous wave laser; duty cycle; micropulse laser; minimally invasive retinal laser treatment; retinal pigment epithelium; temperature increase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Comparison of Continuous-Wave and Micropulse Modulation in Retinal Laser Therapy.Invest Ophthalmol Vis Sci. 2017 Sep 1;58(11):4722-4732. doi: 10.1167/iovs.17-21610. Invest Ophthalmol Vis Sci. 2017. PMID: 28910825
-
Determination of Micropulse Modes with Targeted Damage to the Retinal Pigment Epithelium Using Computer Modeling for the Development of Selective Individual Micropulse Retinal Therapy.Curr Eye Res. 2022 Jan;47(1):107-114. doi: 10.1080/02713683.2021.1962360. Epub 2021 Oct 4. Curr Eye Res. 2022. PMID: 34607475
-
Thermal modelling of micropulsed diode laser retinal photocoagulation.Lasers Surg Med. 1997;20(4):409-15. doi: 10.1002/(sici)1096-9101(1997)20:4<409::aid-lsm6>3.0.co;2-u. Lasers Surg Med. 1997. PMID: 9142680
-
Subthreshold Micropulse Laser for Diabetic Macular Edema: A Review.J Clin Med. 2022 Dec 29;12(1):274. doi: 10.3390/jcm12010274. J Clin Med. 2022. PMID: 36615074 Free PMC article. Review.
-
An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma.Acta Ophthalmol. 2021 Aug;99(5):e621-e653. doi: 10.1111/aos.14661. Epub 2020 Nov 22. Acta Ophthalmol. 2021. PMID: 33222409 Review.
Cited by
-
Functional Outcomes and Safety Profile of Trans-Foveal Subthreshold Micropulse Laser in Persistent Central Serous Chorioretinopathy.Life (Basel). 2023 May 16;13(5):1194. doi: 10.3390/life13051194. Life (Basel). 2023. PMID: 37240839 Free PMC article.
-
Graft cell expansion from hiPSC-RPE strip after transplantation in primate eyes with or without RPE damage.Sci Rep. 2024 May 2;14(1):10044. doi: 10.1038/s41598-024-60895-w. Sci Rep. 2024. PMID: 38698112 Free PMC article.
-
Real-Time Temperature-Controlled Retinal Laser Irradiation in Rabbits.Transl Vis Sci Technol. 2024 Apr 2;13(4):26. doi: 10.1167/tvst.13.4.26. Transl Vis Sci Technol. 2024. PMID: 38639930 Free PMC article.
References
LinkOut - more resources
Full Text Sources

