Structural Design and Synthesis of Novel Cyclic Peptide Inhibitors Targeting Mycobacterium tuberculosis Transcription
- PMID: 36143370
- PMCID: PMC9506182
- DOI: 10.3390/life12091333
Structural Design and Synthesis of Novel Cyclic Peptide Inhibitors Targeting Mycobacterium tuberculosis Transcription
Abstract
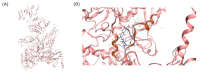
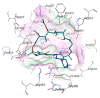
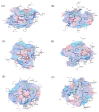
Tuberculosis (TB) remains one of the deadliest infectious diseases in the world. Although several established antitubercular drugs have been found, various factors obstruct efforts to combat this disease due to the existence of drug-resistance (DR) TB strains, the need for lengthy treatment, and the occurrence of side effects from drug-drug interactions. Rifampicin (RIF) is the first line of antitubercular drugs and targets RNA polymerase (RNAP) of Mycobacterium tuberculosis (MTB). Here, RIF blocks the synthesis of long RNA during transcription initiation. The efficacy of RIF is low in DR-TB strains, and the use of RIF leads to various side effects. In this study, novel cyclic peptides were computationally designed as inhibitors of MTB transcription initiation. The designed cyclic peptides were subjected to a virtual screening to generate compounds that can bind to the RIF binding site in MTB RNAP subunit β (RpoB) for obtaining a new potential TB drug with a safe clinical profile. The molecular simulations showed that the cyclic peptides were capable of binding with RpoB mutants, suggesting that they can be possibility utilized for treating DR-TB. Structural modifications were carried out by acetylation and amidation of the N- and C-terminus, respectively, to improve their plasma stability and bioavailability. The modified linear and cyclic peptides were successfully synthesized with a solid-phase peptide synthesis method using Fmoc chemistry, and they were characterized by analytical HPLC, LC-ESI-MS+, and 1H NMR.
Keywords: cyclic peptides; molecular simulation; solid-phase peptide synthesis; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organization . Global Tuberculosis Report 2021. WHO; Geneva, Switzerland: 2021. WHO Report.
-
- Dheda K., Perumal T., Moultrie H., Perumal R., Esmail A., Scott A.J., Udwadia Z., Chang K.C., Peter J., Pooran A., et al. The intersecting pandemics of tuberculosis and COVID-19: Population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir. Med. 2022;10:603–622. doi: 10.1016/S2213-2600(22)00092-3. - DOI - PMC - PubMed
-
- Alipanah N., Jarlsberg L., Miller C., Linh N.N., Falzon D., Jaramillo E., Nahid P. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15:e1002595. doi: 10.1371/journal.pmed.1002595. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

