Upregulation of Spinal miR-155-5p Contributes to Mechanical Hyperalgesia by Promoting Inflammatory Activation of Microglia in Bone Cancer Pain Rats
- PMID: 36143385
- PMCID: PMC9503135
- DOI: 10.3390/life12091349
Upregulation of Spinal miR-155-5p Contributes to Mechanical Hyperalgesia by Promoting Inflammatory Activation of Microglia in Bone Cancer Pain Rats
Abstract
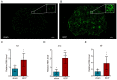
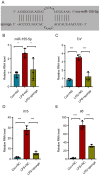
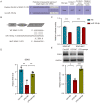
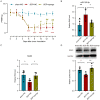
Bone cancer pain (BCP) seriously deteriorates the life quality of patients, but its underlying mechanism is still unclear. Spinal microRNAs might contribute to the development of BCP and the role of microglial activation is controversial. In this study, we established a BCP model by injecting Walker 256 breast carcinoma cells into the tibial intramedullary cavity of rats and significant hyperalgesia was observed in the BCP rats. The lumbar spinal cords were harvested to perform RNA sequencing (RNA-seq), and 31 differentially expressed miRNAs (26 upregulated and 5 downregulated) were identified in the BCP rats. Among them, miR-155-5p was significantly upregulated in the BCP rats. Spinal microglial activation was observed during BCP development. miR-155-5p could be expressed in spinal microglia and was significantly upregulated in microglia treated with lipopolysaccharide (LPS) in vitro. Serum/glucocorticoid regulated kinase family member 3 (Sgk3) was predicted to be the possible downstream target of miR-155-5p and this was confirmed using a dual-luciferase reporter assay in vitro. The inhibition of miR-155-5p restored Sgk3-expression-attenuated microglial activation and alleviated hyperalgesia in the BCP rats. In conclusion, spinal miR-155-5p/Sgk3/microglial activation might play an important role in BCP pathogenesis.
Keywords: Sgk3; bone cancer pain; mechanical hyperalgesia; miR-155-5p; microglia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
miR-155-5p in the spinal cord regulates hypersensitivity in a rat model of bone cancer pain.Mol Pain. 2022 Apr;18:17448069221127811. doi: 10.1177/17448069221127811. Mol Pain. 2022. PMID: 36069070 Free PMC article.
-
miR-32-5p-mediated Dusp5 downregulation contributes to neuropathic pain.Biochem Biophys Res Commun. 2018 Jan 1;495(1):506-511. doi: 10.1016/j.bbrc.2017.11.013. Epub 2017 Nov 3. Biochem Biophys Res Commun. 2018. PMID: 29108992
-
MiR-135-5p Alleviates Bone Cancer Pain by Regulating Astrocyte-Mediated Neuroinflammation in Spinal Cord through JAK2/STAT3 Signaling Pathway.Mol Neurobiol. 2021 Oct;58(10):4802-4815. doi: 10.1007/s12035-021-02458-y. Epub 2021 Jun 27. Mol Neurobiol. 2021. PMID: 34176097
-
Targeting HMGB1-TLR4 signaling by miR-216a-5p elevation alleviates the inflammatory behavioral hypersensitivity.Neurosci Lett. 2021 Aug 10;759:136043. doi: 10.1016/j.neulet.2021.136043. Epub 2021 Jun 10. Neurosci Lett. 2021. PMID: 34118309
-
LncRNA NONRATT009773.2 promotes bone cancer pain progression through the miR-708-5p/CXCL13 axis.Eur J Neurosci. 2022 Feb;55(3):661-674. doi: 10.1111/ejn.15607. Epub 2022 Feb 1. Eur J Neurosci. 2022. PMID: 35075718
Cited by
-
Expression of miR-155 in monocytes of people with migraine: association with phenotype, disease severity and inflammatory profile.J Headache Pain. 2024 Aug 27;25(1):138. doi: 10.1186/s10194-024-01842-y. J Headache Pain. 2024. PMID: 39187749 Free PMC article.
-
The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis.Antioxidants (Basel). 2023 Mar 4;12(3):641. doi: 10.3390/antiox12030641. Antioxidants (Basel). 2023. PMID: 36978889 Free PMC article.
-
MiR-31-5p regulates the neuroinflammatory response via TRAF6 in neuropathic pain.Biol Direct. 2024 Jan 24;19(1):10. doi: 10.1186/s13062-023-00434-1. Biol Direct. 2024. PMID: 38267979 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

