Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat
- PMID: 36143422
- PMCID: PMC9503966
- DOI: 10.3390/life12091386
Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat
Abstract
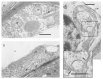
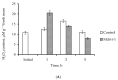
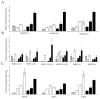
Mitochondria play a key role in providing energy to cells. These organelles are constantly undergoing dynamic processes of fusion and fission that change in stressful conditions. The role of mitochondrial fusion in wheat root cells was studied using Mdivi-1, an inhibitor of the mitochondrial fragmentation protein Drp1. The effect of the inhibitor was studied on mitochondrial dynamics in the roots of wheat seedlings subjected to a wounding stress, simulated by excision. Treatment of the stressed roots with the inhibitor increased the size of the mitochondria, enhanced their functional activity, and elevated their membrane potentials. Mitochondrial fusion was accompanied by a decrease in ROS formation and associated cell damage. Exposure to Mdivi-1 also upregulated genes encoding the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and an energy sensor AMP-dependent protein sucrose non-fermenting-related kinase (SnRK1), suggesting that mitochondrial fusion is associated with a general activation of energy metabolism. Controlling mitochondrial fusion rates could change the physiology of wheat plants by altering the energy status of the cell and helping to mitigate the effects of stress.
Keywords: energy status; fusion; mitochondria; redox metabolism; wheat; wounding.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity.Hum Mol Genet. 2019 Jan 15;28(2):177-199. doi: 10.1093/hmg/ddy335. Hum Mol Genet. 2019. PMID: 30239719 Free PMC article.
-
Pleiotropic effects of mdivi-1 in altering mitochondrial dynamics, respiration, and autophagy in cardiomyocytes.Redox Biol. 2020 Sep;36:101660. doi: 10.1016/j.redox.2020.101660. Epub 2020 Jul 26. Redox Biol. 2020. PMID: 32750667 Free PMC article.
-
Mitochondrial fission - a drug target for cytoprotection or cytodestruction?Pharmacol Res Perspect. 2016 Apr 21;4(3):e00235. doi: 10.1002/prp2.235. eCollection 2016 Jun. Pharmacol Res Perspect. 2016. PMID: 27433345 Free PMC article. Review.
-
To mdivi-1 or not to mdivi-1: Is that the question?Dev Neurobiol. 2017 Nov;77(11):1260-1268. doi: 10.1002/dneu.22519. Epub 2017 Aug 30. Dev Neurobiol. 2017. PMID: 28842943 Free PMC article. Review.
-
Drp1-dependent mitochondrial fission mediates corneal injury induced by alkali burn.Free Radic Biol Med. 2021 Nov 20;176:149-161. doi: 10.1016/j.freeradbiomed.2021.09.019. Epub 2021 Sep 22. Free Radic Biol Med. 2021. PMID: 34562609
Cited by
-
Nitric Oxide Induces Autophagy in Triticum aestivum Roots.Antioxidants (Basel). 2023 Aug 22;12(9):1655. doi: 10.3390/antiox12091655. Antioxidants (Basel). 2023. PMID: 37759958 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

