Recent Advances in the Study of Gas Vesicle Proteins and Application of Gas Vesicles in Biomedical Research
- PMID: 36143491
- PMCID: PMC9501494
- DOI: 10.3390/life12091455
Recent Advances in the Study of Gas Vesicle Proteins and Application of Gas Vesicles in Biomedical Research
Abstract
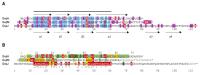
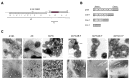
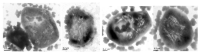
The formation of gas vesicles has been investigated in bacteria and haloarchaea for more than 50 years. These air-filled nanostructures allow cells to stay at a certain height optimal for growth in their watery environment. Several gvp genes are involved and have been studied in Halobacterium salinarum, cyanobacteria, Bacillus megaterium, and Serratia sp. ATCC39006 in more detail. GvpA and GvpC form the gas vesicle shell, and additional Gvp are required as minor structural proteins, chaperones, an ATP-hydrolyzing enzyme, or as gene regulators. We analyzed the Gvp proteins of Hbt. salinarum with respect to their protein-protein interactions, and developed a model for the formation of these nanostructures. Gas vesicles are also used in biomedical research. Since they scatter waves and produce ultrasound contrast, they could serve as novel contrast agent for ultrasound or magnetic resonance imaging. Additionally, gas vesicles were engineered as acoustic biosensors to determine enzyme activities in cells. These applications are based on modifications of the surface protein GvpC that alter the mechanical properties of the gas vesicles. In addition, gas vesicles have been decorated with GvpC proteins fused to peptides of bacterial or viral pathogens and are used as tools for vaccine development.
Keywords: Halobacterium salinarum; acoustic biosensors; gas vesicles; protein nanostructures; vaccine development.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Haloarchaea and the formation of gas vesicles.Life (Basel). 2015 Feb 2;5(1):385-402. doi: 10.3390/life5010385. Life (Basel). 2015. PMID: 25648404 Free PMC article. Review.
-
Analysis of gas vesicle gene expression in Haloferax mediterranei reveals that GvpA and GvpC are both gas vesicle structural proteins.J Biol Chem. 1993 May 5;268(13):9329-36. J Biol Chem. 1993. PMID: 7683649
-
Accessory Gvp Proteins Form a Complex During Gas Vesicle Formation of Haloarchaea.Front Microbiol. 2020 Nov 12;11:610179. doi: 10.3389/fmicb.2020.610179. eCollection 2020. Front Microbiol. 2020. PMID: 33281806 Free PMC article.
-
The characterization of the nv-gvpACNOFGH gene cluster involved in gas vesicle formation in Natronobacterium vacuolatum.Arch Microbiol. 1997 Jul;168(1):24-32. doi: 10.1007/s002030050465. Arch Microbiol. 1997. PMID: 9211710
-
Regulation of gas vesicle formation in halophilic archaea.J Mol Microbiol Biotechnol. 2002 May;4(3):175-81. J Mol Microbiol Biotechnol. 2002. PMID: 11931543 Review.
Cited by
-
Advances in the application of gas vesicles in medical imaging and disease treatment.J Biol Eng. 2024 Jul 23;18(1):41. doi: 10.1186/s13036-024-00426-3. J Biol Eng. 2024. PMID: 39044273 Free PMC article. Review.
-
Genetically engineered gas vesicle proteins with proliferative potential for synergistic targeted tumor therapy.RSC Adv. 2025 Jan 2;15(1):157-166. doi: 10.1039/d4ra07532c. eCollection 2025 Jan 2. RSC Adv. 2025. PMID: 39758902 Free PMC article.
-
Exploiting Sound for Emerging Applications of Extracellular Vesicles.Nano Res. 2024 Feb;17(2):462-475. doi: 10.1007/s12274-023-5840-6. Epub 2023 Jul 1. Nano Res. 2024. PMID: 38712329 Free PMC article.
-
Low Salt Influences Archaellum-Based Motility, Glycerol Metabolism, and Gas Vesicles Biogenesis in Halobacterium salinarum.Microorganisms. 2022 Dec 10;10(12):2442. doi: 10.3390/microorganisms10122442. Microorganisms. 2022. PMID: 36557695 Free PMC article.
-
Excavation of acoustic nanostructures biosynthesis gene clusters by combinatorial strategy.Adv Biotechnol (Singap). 2025 May 15;3(2):15. doi: 10.1007/s44307-025-00069-5. Adv Biotechnol (Singap). 2025. PMID: 40372536 Free PMC article.
References
-
- Walsby A.E. The permeability of blue-green algal gas-vacuole membranes to gas. Proc. R. Soc. Lond B Biol. Sci. 1969;173:235–255. doi: 10.1098/rspb.1969.0049. - DOI
-
- Thomas R.H., Walsby A.E. Buoyancy regulation in a strain of Microcystis. J. Gen. Microbiol. 1985;131:799–809. doi: 10.1099/00221287-131-4-799. - DOI
-
- Oliver R.L., Walsby A.E. Direct evidence for the role of light-mediated gas vesicle collapse in the buoyancy regulation of Anabaena flos-aquae (Cyanobacteria) Limnol. Oceanogr. 1984;29:879–886. doi: 10.4319/lo.1984.29.4.0879. - DOI
-
- Walsby A.E., Mcallister G.K. Buoyancy regulation by Microcystis in Lake Okaro. N. Z. J. Mar. Fresh. 1987;21:521–524. doi: 10.1080/00288330.1987.9516249. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

