Identification of Suitable Drug Combinations for Treating COVID-19 Using a Novel Machine Learning Approach: The RAIN Method
- PMID: 36143492
- PMCID: PMC9505329
- DOI: 10.3390/life12091456
Identification of Suitable Drug Combinations for Treating COVID-19 Using a Novel Machine Learning Approach: The RAIN Method
Abstract
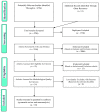
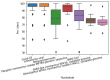
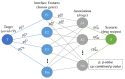
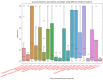
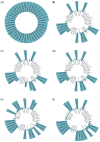
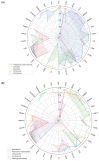
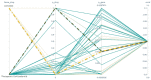
COVID-19 affects several human genes, each with its own p-value. The combination of drugs associated with these genes with small p-values may lead to an estimation of the combined p-value between COVID-19 and some drug combinations, thereby increasing the effectiveness of these combinations in defeating the disease. Based on human genes, we introduced a new machine learning method that offers an effective drug combination with low combined p-values between them and COVID-19. This study follows an improved approach to systematic reviews, called the Systematic Review and Artificial Intelligence Network Meta-Analysis (RAIN), registered within PROSPERO (CRD42021256797), in which, the PRISMA criterion is still considered. Drugs used in the treatment of COVID-19 were searched in the databases of ScienceDirect, Web of Science (WoS), ProQuest, Embase, Medline (PubMed), and Scopus. In addition, using artificial intelligence and the measurement of the p-value between human genes affected by COVID-19 and drugs that have been suggested by clinical experts, and reported within the identified research papers, suitable drug combinations are proposed for the treatment of COVID-19. During the systematic review process, 39 studies were selected. Our analysis shows that most of the reported drugs, such as azithromycin and hydroxyl-chloroquine on their own, do not have much of an effect on the recovery of COVID-19 patients. Based on the result of the new artificial intelligence, on the other hand, at a significance level of less than 0.05, the combination of the two drugs therapeutic corticosteroid + camostat with a significance level of 0.02, remdesivir + azithromycin with a significance level of 0.03, and interleukin 1 receptor antagonist protein + camostat with a significance level 0.02 are considered far more effective for the treatment of COVID-19 and are therefore recommended. Additionally, at a significance level of less than 0.01, the combination of interleukin 1 receptor antagonist protein + camostat + azithromycin + tocilizumab + oseltamivir with a significance level of 0.006, and the combination of interleukin 1 receptor antagonist protein + camostat + chloroquine + favipiravir + tocilizumab7 with corticosteroid + camostat + oseltamivir + remdesivir + tocilizumab at a significant level of 0.009 are effective in the treatment of patients with COVID-19 and are also recommended. The results of this study provide sets of effective drug combinations for the treatment of patients with COVID-19. In addition, the new artificial intelligence used in the RAIN method could provide a forward-looking approach to clinical trial studies, which could also be used effectively in the treatment of diseases such as cancer.
Keywords: COVID-19; RAIN method; drugs combinations; machine learning; network meta-analysis; treatment of patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
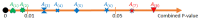


References
-
- Salari N., Khazaie H., Hosseinian-Far A., Ghasemi H., Mohammadi M., Shohaimi S., Daneshkhah A., Khaledi-Paveh B., Hosseinian-Far M. The prevalence of sleep disturbances among physicians and nurses facing the COVID-19 patients: A systematic review and meta-analysis. Glob. Health. 2020;16:92. doi: 10.1186/s12992-020-00620-0. - DOI - PMC - PubMed
-
- Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., Gordeev I.G., Ilin A.P., Karapetian R.N., Kravchenko D.V., et al. AVIFAVIR for Treatment of Patients with Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021;73:531–534. doi: 10.1093/cid/ciaa1176. - DOI - PMC - PubMed
-
- Li C., Luo F., Liu C., Xiong N., Xu Z., Zhang W., Yang M., Wang Y., Liu D., Yu C., et al. Effect of a genetically engineered interferon-alpha versus traditional interferon-alpha in the treatment of moderate-to-severe COVID-19: A randomised clinical trial. Ann. Med. 2021;53:391–401. doi: 10.1080/07853890.2021.1890329. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

