Refined Lord-Shulman Theory for 1D Response of Skin Tissue under Ramp-Type Heat
- PMID: 36143604
- PMCID: PMC9505323
- DOI: 10.3390/ma15186292
Refined Lord-Shulman Theory for 1D Response of Skin Tissue under Ramp-Type Heat
Abstract
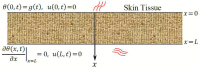
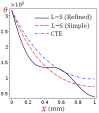
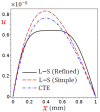
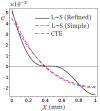
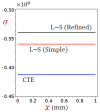
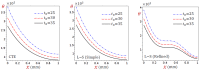
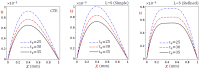
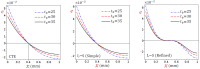
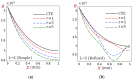
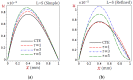
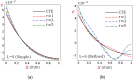
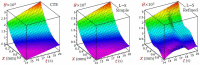
In this article, we present a mathematical model of thermoelastic skin tissue based on a refined Lord-Shulman heat conduction theory. A small thickness of skin tissue is considered to be one-dimensional with mechanical clamped surfaces. In addition, the skin tissue's outer surface is subjected to ramp-type heating while its inner surface is adiabatic. A simple Lord-Shulman theory, as well as the classical coupled thermoelasticity, are also applied in this article. Laplace transform techniques and their inversions are calculated to return to the time domain. Numerical outcomes are represented graphically to discuss the significant impacts on the temperature, dilatation, displacement, and stress distributions. Such results provide a more comprehensive and better insight for understanding the behavior of skin tissue during the temperature distribution of a specific boundary condition.
Keywords: bio-thermoelasticity; ramp-type heating; refined L–S model; skin tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Refined Dual-Phase-Lag Theory for the 1D Behavior of Skin Tissue under Ramp-Type Heating.Materials (Basel). 2023 Mar 17;16(6):2421. doi: 10.3390/ma16062421. Materials (Basel). 2023. PMID: 36984301 Free PMC article.
-
A Modified Two-Relaxation Thermoelastic Model for a Thermal Shock of Rotating Infinite Medium.Materials (Basel). 2022 Dec 18;15(24):9056. doi: 10.3390/ma15249056. Materials (Basel). 2022. PMID: 36556861 Free PMC article.
-
Modeling of One-Dimensional Thermoelastic Dual-Phase-Lag Skin Tissue Subjected to Different Types of Thermal Loading.Sci Rep. 2020 Feb 25;10(1):3399. doi: 10.1038/s41598-020-60342-6. Sci Rep. 2020. PMID: 32099007 Free PMC article.
-
Thermal-stress analysis of a damaged solid sphere using hyperbolic two-temperature generalized thermoelasticity theory.Sci Rep. 2021 Jan 27;11(1):2289. doi: 10.1038/s41598-021-82127-1. Sci Rep. 2021. PMID: 33504922 Free PMC article.
-
The effects of thermal and mechanical material properties on tumorous tissue during hyperthermia treatment.J Therm Biol. 2020 Aug;92:102649. doi: 10.1016/j.jtherbio.2020.102649. Epub 2020 Jul 19. J Therm Biol. 2020. PMID: 32888556
Cited by
-
Refined Dual-Phase-Lag Theory for the 1D Behavior of Skin Tissue under Ramp-Type Heating.Materials (Basel). 2023 Mar 17;16(6):2421. doi: 10.3390/ma16062421. Materials (Basel). 2023. PMID: 36984301 Free PMC article.
-
A Modified Two-Relaxation Thermoelastic Model for a Thermal Shock of Rotating Infinite Medium.Materials (Basel). 2022 Dec 18;15(24):9056. doi: 10.3390/ma15249056. Materials (Basel). 2022. PMID: 36556861 Free PMC article.
References
-
- Li X., Qin Q.H., Tian X. Thermomechanical response of porous biological tissue based on local thermal non-equilibrium. J. Therm. Stress. 2019;42:1481–1498. doi: 10.1080/01495739.2019.1660599. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

