Delayed Adverse Events after Procedural Sedation in Pediatric Patients with Hematologic Malignancies
- PMID: 36143885
- PMCID: PMC9501634
- DOI: 10.3390/medicina58091208
Delayed Adverse Events after Procedural Sedation in Pediatric Patients with Hematologic Malignancies
Abstract
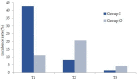
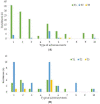
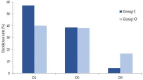
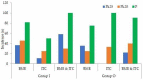
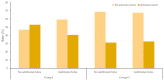
Background and objectives: Procedural sedation for bone marrow examination (BME) and intrathecal chemotherapy (ITC) is necessary for pediatric patients with hematological malignancies. There has been no report on adverse events after discharge from the recovery room. This retrospective study evaluated the types and incidences of delayed adverse events among pediatric patients scheduled for BME or ITC under deep sedation in a single center for 3 years. Materials and Methods: The patients were divided into two groups: inpatients (group I) and outpatients (group O). All patients were managed during the procedures and the recovery period. In total, 10 adverse events were assessed; these occurred 2 h (T1, acute), 12 h (T2, early), and 24 h (T3, delayed) after the procedure. The duration of each adverse event was also recorded and was classified as 2 h (D1), 12 h (D2), or 24 h (D3). The data of 263 patients (147 inpatients and 116 outpatients) who met the inclusion criteria were analyzed. Results: The overall incidence of adverse events was statistically significant difference: 48.3% in group I and 33.6% in group O (p = 0.011). The rates of adverse events at T1 and T2 were significantly different between groups I and O (42.8% vs. 11.2% and 7.5% vs. 20.7%, respectively) (p < 0.001). The adverse events were mostly of D1 or D2 duration in both groups. Patients with a higher proportion of ketamine in a propofol−ketamine mixture had a significantly higher proportion of adverse events at T1 (34.6%), as compared with those with a mixture with a lower proportion of ketamine (21.1%) or propofol alone (17.9%) (p = 0.012). Conclusions: The most common adverse events were dizziness or headache; typically, they did not last longer than 12 h. The propofol-ketamine combination with a higher proportion of ketamine seems to produce more adverse events within 2 h after the procedure. Nevertheless, all sedative types appear safe to use without additional management.
Keywords: adverse event; hematological malignancies; ketamine; pediatric; procedural sedation; propofol.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Pediatric Procedural Sedation Using the Combination of Ketamine and Propofol Outside of the Emergency Department: A Report From the Pediatric Sedation Research Consortium.Pediatr Crit Care Med. 2017 Aug;18(8):e356-e363. doi: 10.1097/PCC.0000000000001246. Pediatr Crit Care Med. 2017. PMID: 28650904 Free PMC article.
-
Randomized clinical trial of propofol versus ketamine for procedural sedation in the emergency department.Acad Emerg Med. 2010 Jun;17(6):604-11. doi: 10.1111/j.1553-2712.2010.00776.x. Acad Emerg Med. 2010. PMID: 20624140 Clinical Trial.
-
Adverse Events During a Randomized Trial of Ketamine Versus Co-Administration of Ketamine and Propofol for Procedural Sedation in a Pediatric Emergency Department.J Emerg Med. 2017 Jul;53(1):1-9. doi: 10.1016/j.jemermed.2017.03.024. Epub 2017 Apr 19. J Emerg Med. 2017. PMID: 28433211 Clinical Trial.
-
Combination ketamine and propofol for procedural sedation and analgesia.Pharmacotherapy. 2007 Nov;27(11):1588-98. doi: 10.1592/phco.27.11.1588. Pharmacotherapy. 2007. PMID: 17963466 Review.
-
Ketofol for Procedural Sedation and Analgesia in the Pediatric Population.Pediatr Emerg Care. 2022 Jan 1;38(1):28-33. doi: 10.1097/PEC.0000000000002599. Pediatr Emerg Care. 2022. PMID: 34986578 Review.
Cited by
-
A comparative study on the efficacy and safety of propofol combined with different doses of alfentanil in gastroscopy: a randomized controlled trial.J Anesth. 2023 Apr;37(2):201-209. doi: 10.1007/s00540-022-03145-5. Epub 2022 Dec 8. J Anesth. 2023. PMID: 36482231 Clinical Trial.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

