Advances in Cancer Diagnosis: Bio-Electrochemical and Biophysical Characterizations of Cancer Cells
- PMID: 36144024
- PMCID: PMC9504238
- DOI: 10.3390/mi13091401
Advances in Cancer Diagnosis: Bio-Electrochemical and Biophysical Characterizations of Cancer Cells
Abstract
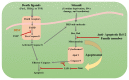
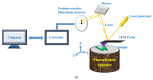
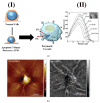
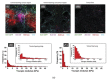
Cancer is a worldwide leading cause of death, and it is projected that newly diagnosed cases globally will reach 27.5 million each year by 2040. Cancers (malignant tumors), unlike benign tumors are characterized by structural and functional dedifferentiation (anaplasia), breaching of the basement membrane, spreading to adjacent tissues (invasiveness), and the capability to spread to distant sites (metastasis). In the cancer biology research field, understanding and characterizing cancer metastasis as well as features of cell death (apoptosis) is considered a technically challenging subject of study and clinically is very critical and necessary. Therefore, in addition to the cytochemical methods traditionally used, novel biophysical and bioelectrochemical techniques (e.g., cyclic voltammetry and electrochemical impedance spectroscopy), atomic force microscopy, and electron microscopic methods are increasingly being deployed to better understand these processes. Implementing those methods at the preclinical level enables the rapid screening of new anticancer drugs with understanding of their central mechanism for cancer therapy. In this review, principles and basic concepts of new techniques suggested for metastasis, and apoptosis examinations for research purposes are introduced, along with examples of each technique. From our recommendations, the privilege of combining the bio-electrochemical and biosensing techniques with the conventional cytochemical methods either for research or for biomedical diagnosis should be emphasized.
Keywords: apoptosis; atomic force microscopy; cancer biology; electrochemical biosensors; electron microscopy; in-vitro assessment; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications.Sensors (Basel). 2021 Oct 1;21(19):6578. doi: 10.3390/s21196578. Sensors (Basel). 2021. PMID: 34640898 Free PMC article. Review.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
-
Label-Free Bioelectrochemical Methods for Evaluation of Anticancer Drug Effects at a Molecular Level.Sensors (Basel). 2020 Mar 25;20(7):1812. doi: 10.3390/s20071812. Sensors (Basel). 2020. PMID: 32218227 Free PMC article. Review.
-
Atomic force microscopy as an imaging tool to study the bio/nonbio complexes.J Microsc. 2020 Dec;280(3):241-251. doi: 10.1111/jmi.12936. Epub 2020 Jun 17. J Microsc. 2020. PMID: 32519330
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
Cited by
-
Non-enzymatic disposable electrochemical sensors based on CuO/Co3O4@MWCNTs nanocomposite modified screen-printed electrode for the direct determination of urea.Sci Rep. 2023 Feb 4;13(1):2034. doi: 10.1038/s41598-023-28930-4. Sci Rep. 2023. PMID: 36739320 Free PMC article.
-
Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives.Sensors (Basel). 2022 Oct 5;22(19):7539. doi: 10.3390/s22197539. Sensors (Basel). 2022. PMID: 36236638 Free PMC article. Review.
-
Transformative insights in breast cancer: review of atomic force microscopy applications.Discov Oncol. 2025 Feb 28;16(1):256. doi: 10.1007/s12672-025-02003-6. Discov Oncol. 2025. PMID: 40021496 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

