Fabrication of Concave Microwells and Their Applications in Micro-Tissue Engineering: A Review
- PMID: 36144178
- PMCID: PMC9505614
- DOI: 10.3390/mi13091555
Fabrication of Concave Microwells and Their Applications in Micro-Tissue Engineering: A Review
Abstract
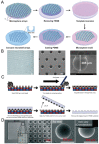
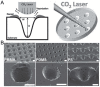
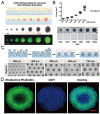
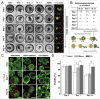
At present, there is an increasing need to mimic the in vivo micro-environment in the culture of cells and tissues in micro-tissue engineering. Concave microwells are becoming increasingly popular since they can provide a micro-environment that is closer to the in vivo environment compared to traditional microwells, which can facilitate the culture of cells and tissues. Here, we will summarize the fabrication methods of concave microwells, as well as their applications in micro-tissue engineering. The fabrication methods of concave microwells include traditional methods, such as lithography and etching, thermal reflow of photoresist, laser ablation, precision-computerized numerical control (CNC) milling, and emerging technologies, such as surface tension methods, the deformation of soft membranes, 3D printing, the molding of microbeads, air bubbles, and frozen droplets. The fabrication of concave microwells is transferring from professional microfabrication labs to common biochemical labs to facilitate their applications and provide convenience for users. Concave microwells have mostly been used in organ-on-a-chip models, including the formation and culture of 3D cell aggregates (spheroids, organoids, and embryoids). Researchers have also used microwells to study the influence of substrate topology on cellular behaviors. We will briefly review their applications in different aspects of micro-tissue engineering and discuss the further applications of concave microwells. We believe that building multiorgan-on-a-chip by 3D cell aggregates of different cell lines will be a popular application of concave microwells, while integrating physiologically relevant molecular analyses with the 3D culture platform will be another popular application in the near future. Furthermore, 3D cell aggregates from these biosystems will find more applications in drug screening and xenogeneic implantation.
Keywords: 3D printing; CNC milling; cellular behavior; embryoid; etching; lithography; organoid; photoresist reflow; spheroid; surface tension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















Similar articles
-
Concave Microwell Formation Induced by PDMS Water Vapor Permeability for Spheroid Generation.Micromachines (Basel). 2024 Dec 14;15(12):1496. doi: 10.3390/mi15121496. Micromachines (Basel). 2024. PMID: 39770249 Free PMC article.
-
A Paired Bead and Magnet Array for Molding Microwells with Variable Concave Geometries.J Vis Exp. 2018 Jan 28;(131):55548. doi: 10.3791/55548. J Vis Exp. 2018. PMID: 29443026 Free PMC article.
-
Enhanced oxygen permeability in membrane-bottomed concave microwells for the formation of pancreatic islet spheroids.Acta Biomater. 2018 Jan;65:185-196. doi: 10.1016/j.actbio.2017.10.045. Epub 2017 Oct 31. Acta Biomater. 2018. PMID: 29101017
-
A Thorough Review of Emerging Technologies in Micro- and Nanochannel Fabrication: Limitations, Applications, and Comparison.Micromachines (Basel). 2024 Oct 21;15(10):1274. doi: 10.3390/mi15101274. Micromachines (Basel). 2024. PMID: 39459148 Free PMC article. Review.
-
Organoid and Spheroid Tumor Models: Techniques and Applications.Cancers (Basel). 2021 Feb 19;13(4):874. doi: 10.3390/cancers13040874. Cancers (Basel). 2021. PMID: 33669619 Free PMC article. Review.
Cited by
-
Breaking the clean room barrier: exploring low-cost alternatives for microfluidic devices.Front Bioeng Biotechnol. 2023 Apr 27;11:1176557. doi: 10.3389/fbioe.2023.1176557. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37180035 Free PMC article. Review.
-
Transforming Drug Discovery with Miniaturized Predictive Tissue Models.Micromachines (Basel). 2025 Mar 3;16(3):299. doi: 10.3390/mi16030299. Micromachines (Basel). 2025. PMID: 40141910 Free PMC article.
-
Simple and Rapid Method of Microwell Array Fabrication for Drug Testing on 3D Cancer Spheroids.ACS Omega. 2024 Apr 5;9(15):16949-16958. doi: 10.1021/acsomega.3c05873. eCollection 2024 Apr 16. ACS Omega. 2024. PMID: 38645317 Free PMC article.
-
Design, Fabrication, and Applications of SERS Substrates for Food Safety Detection: Review.Micromachines (Basel). 2023 Jun 30;14(7):1343. doi: 10.3390/mi14071343. Micromachines (Basel). 2023. PMID: 37512654 Free PMC article. Review.
-
Construction of tumor organoids and their application to cancer research and therapy.Theranostics. 2024 Jan 12;14(3):1101-1125. doi: 10.7150/thno.91362. eCollection 2024. Theranostics. 2024. PMID: 38250041 Free PMC article. Review.
References
-
- Zhang B., Korolj A., Lai B.F.L., Radisic M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018;3:257–278. doi: 10.1038/s41578-018-0034-7. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

