The Improved Biocontrol Agent, F1-35, Protects Watermelon against Fusarium Wilt by Triggering Jasmonic Acid and Ethylene Pathways
- PMID: 36144312
- PMCID: PMC9501610
- DOI: 10.3390/microorganisms10091710
The Improved Biocontrol Agent, F1-35, Protects Watermelon against Fusarium Wilt by Triggering Jasmonic Acid and Ethylene Pathways
Abstract
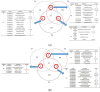
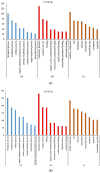
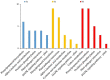
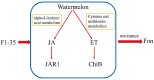
Watermelon Fusarium wilt, caused by Fusarium oxysporum f. sp. niveum (FON), is one of the most important diseases, and has become a major limiting factor to watermelon production worldwide. Previous research has found that the improved biocontrol agent, F1-35, had a high control efficiency to watermelon Fusarium wilt. In this study, the control efficiency of F1-35 to watermelon Fusarium wilt was firstly tested, and the control efficiency was 61.7%. Then, we investigated the mode of action of F1-35 in controlling watermelon Fusarium wilt. Using a pairing assay, we found that F1-35 did not inhibit the normal growth of FON. To know more about the interaction between F1-35 and watermelon root, the protein expressions of roots after 12, 24, and 48 h post-inoculation were examined. A total of 1109 differentially expressed proteins were obtained. KEGG analysis found that the most differentially expressed proteins occurred in alpha-linolenic acid metabolism, cysteine and methionine metabolism, plant-pathogen interaction, and the MAPK signaling pathway to the plant. A further analysis of differentially expressed proteins showed that F1-35 triggered the jasmonic acid and ethylene pathways in watermelon. To validate our results, the qRT-PCR was used to analyze the gene expression levels of PAL, LOX1, and CTR1. The gene expression results showed that those genes, which were positive correlated with the JA pathway, were up-expressed, including PAL and LOX1, and the negative associated gene, CTR1, was down-expressed. In conclusion, the improved biocontrol agent, F1-35, improves the resistance of watermelons to FON by triggering the JA and ET pathways.
Keywords: JA and ET pathway; biological control; proteomes; qRT-PCR; watermelon Fusarium wilt.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Bacterial extracellular biomolecules-derived multimodal manganese nanoparticles control watermelon Fusarium wilt by dysregulating fusaric acid biosynthesis pathway and precise tuning of rhizosphere metabolome.J Nanobiotechnology. 2025 Jun 18;23(1):452. doi: 10.1186/s12951-025-03492-x. J Nanobiotechnology. 2025. PMID: 40533729 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Identification of Fusarium oxysporum f. sp. lactucae Race 1 as the Causal Agent of Lettuce Fusarium Wilt in Greece, Commercial Cultivars' Susceptibility, and Temporal Expression of Defense-Related Genes.Microorganisms. 2023 Apr 20;11(4):1082. doi: 10.3390/microorganisms11041082. Microorganisms. 2023. PMID: 37110505 Free PMC article.
-
Development of PCR-Based Race-Specific Markers for Differentiation of Indian Fusarium oxysporum f. sp. cubense, the Causal Agent of Fusarium Wilt in Banana.J Fungi (Basel). 2022 Jan 5;8(1):53. doi: 10.3390/jof8010053. J Fungi (Basel). 2022. PMID: 35049993 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
Cited by
-
Potential of Streptomyces rochei G-6 for Biocontrol of Cucumber Wilt Disease and Growth Enhancement.J Fungi (Basel). 2024 Dec 20;10(12):885. doi: 10.3390/jof10120885. J Fungi (Basel). 2024. PMID: 39728381 Free PMC article.
-
Tissue-Specific Hormone Signalling and Defence Gene Induction in an In Vitro Assembly of the Rapeseed Verticillium Pathosystem.Int J Mol Sci. 2023 Jun 22;24(13):10489. doi: 10.3390/ijms241310489. Int J Mol Sci. 2023. PMID: 37445666 Free PMC article.
References
-
- Massaccesi G., Romero M.C., Cazau M.C., Bucsinszky A.M. Cadmium removal capacities of filamentous soil fungi isolated from industrially polluted sediments, in La Plata (Argentina) World J. Microbiol. Biot. 2002;18:817–820. doi: 10.1023/A:1021282718440. - DOI
-
- Ortega-Larrocea M.D., Xoconostle-Cazares B., Maldonado-Mendoza I.E., Carrillo-Gonzalez R., Hernandez-Hernandez J., Garduno M.D., Lopez-Meyer M., Gomez-Flores L., Gonzalez-Chavez M.D.A. Plant and fungal biodiversity from metal mine wastes under remediation at Zimapan, Hidalgo, Mexico. Environ. Pollut. 2010;158:1922–1931. doi: 10.1016/j.envpol.2009.10.034. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

